Diabetes Device Innovation: Changing Current Treatment Algorithms

By Manya Aggarwal, Decision Resources Group
Diabetes is a chronic disease that, according to the World Health Organization (WHO), affected almost 9 percent of adults globally in 2014. The incidence of this disease is expected to nearly double by 2030 as contributing factors, such as obesity rates, continue to rise around the world.
The prevalence of the condition results in a very large market for both drug delivery and diabetes-monitoring devices. Decision Resource Group’s report on diabetes devices estimates that this market will grow at a rate of over 10 percent annually in the next 10 years. This presents a very attractive opportunity for manufacturers in this space, especially since there is a lot of room for improvement in the treatment and management of the disease.
Therefore, there is strong interest in new innovations that would make diabetes easier to manage and control, thereby allowing patients worldwide to lead more normal lives. This interest has precipitated a race among device manufacturers to spearhead innovations in order to maximize revenues in this very lucrative space.
Diabetes Treatment Algorithms
Type 2 diabetes — also known as adult onset diabetes — constitutes the majority of cases diagnosed around the world, while type 1 diabetes accounts for a significantly smaller proportion of cases. The treatment algorithms differ significantly between type 1 and type 2 diabetes, as shown in the figures below:

Type 1 diabetes, also known as juvenile diabetes, is characterized by deficient insulin production and requires daily administration of insulin. The first line of treatment is basal insulin, whose effects can last anywhere between 24 and36 hours. If this dosage is not sufficient to keep blood glucose levels within the stipulated range, then the patient is injected with an additional dose of bolus insulin at meal times, in addition to basal insulin.
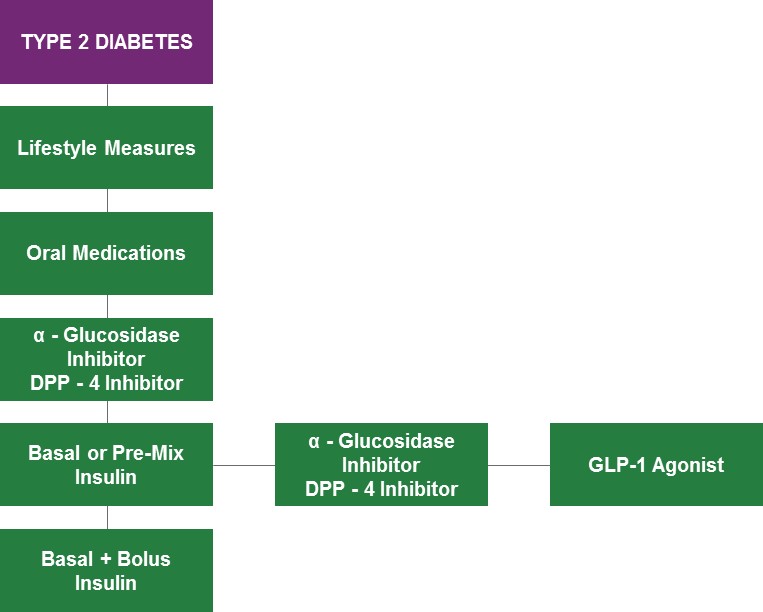
Type 2 diabetes, on the other hand, is characterized by the body’s ineffective use of insulin. Symptoms are usually less marked than type 1 diabetes. This is the reason that lifestyle changes are usually the first line of treatment: to stimulate the pancreas to naturally produce insulin. If this is insufficient, then a second line of treatment puts patients on oral antidiabetic medications. These medications include metformin, sulfonyurea, or thiazolidinedione pills.
If a combination of lifestyle changes and medication is still not sufficient to control the condition, then the patient is either put on DPP-4 inhibitor therapy or alpha glucosidase inhibitor therapy. It is only if all these lines of treatment prove ineffective that the patient has to start insulin therapy, which may or may not need to be combined with GLP-1 agonists or DPP-4 inhibitors. This is the stage at which type 2 diabetics have to frequently monitor their blood glucose levels and require regular insulin injections, much like type 1 diabetics.
The need for improved treatment algorithms to manage both type 1 and type 2 diabetes arises from the significant shortcomings associated with current options. For all type 1 diabetics, and some type 2 diabetics on insulin therapy, very active user monitoring is required to properly regulate blood glucose levels. This involves diabetics counting calories and calculating the amount of insulin needed around mealtimes in order to inject themselves with the correct dosage. Any mistake in this calculation can expose the patient to the risk of hypoglycemia, which can lead to mental confusion, unconsciousness, and in extreme cases, even death.
Here, I will discuss two impending innovations which, upon release, will help address some of the shortcomings associated with current diabetes treatments options.
The Artificial Pancreas
An “artificial pancreas” system would consist of a continuous glucose monitoring (CGM) device and an insulin pump, which would be linked together through the threshold suspend system. This device would require no external user input to function. Essentially, the CGM unit would detect low blood glucose levels automatically and use the threshold suspend system to alert the insulin pump unit to stop dispensing insulin. Similarly, if blood glucose levels were to rise above normal amounts, the threshold suspend system would alert the insulin pump to release higher doses of insulin to regulate blood glucose levels.
Medtronic is leading innovation in this space and has already launched a product in Europe called the MiniMed 640G. This device can detect low blood glucose levels and suspend insulin delivery, but it cannot automatically release higher doses of insulin in cases where high blood glucose levels are detected. The MiniMed 640G represents an important step in the creation of a true artificial pancreas, but further development is necessary to overcome its limitations. Medtronic also has some “hybrid closed loop” products in research, and a more advanced MiniMed device could be released to market as early as 2017 or 2018.
While this device is not expected to change the current treatment algorithm, it would nevertheless remove the necessity for very active user input by essentially making the process entirely automatic. Severe type 1 diabetics would be able to forget about their diabetes and lead normal lives without calculating calories every meal of the day.
The GLP-1 Agonist Implant
The ITCA 650 drug delivery implant would accomplish the same goal for type 2 diabetics. Currently under development by Intarcia Therapeutics, the implant is a small, osmotic pump that is intended to be placed subdermally in the abdomen to continuously release the GLP-1 agonist Exenatide. This product is currently in Phase III clinical trials and is expected to enter the market in 2017.
This delivery device has already shown significant potential in trials. It outperformed Merck’s comparable DPP-4 inhibitor drug Januvia at lowering blood glucose levels, as well as reducing body weight. It is also easier to use than Novo Nordisk’s injectable GLP-1 drug Victoza, since it only needs to be replaced once a year, while Victoza requires daily or weekly injections.
This device would alter the treatment algorithm for type 2 diabetes, becoming a viable third-line treatment option after lifestyle measures and oral medications. It would reduce the burden of monitoring and compliance on the patient, since it only needs replacement around once a year. Due to the positive results demonstrated in clinical trials, it also has the potential to displace other injectable DPP-4 inhibitors in the line of treatment. However, if the diabetes progressively gets worse despite the implant’s usage, it could be supplemented with DPP-4 inhibitors, other GLP-1 agonists, and finally insulin therapy. The ITCA 650 could potentially also delay the use of insulin therapy compared to the current treatment algorithm.
Conclusions
Type 1 and type 2 diabetics around the world are seeking ways to escape the problems posed by the current treatment algorithms for managing this disease. Device manufacturers must devise innovations that minimize or remove the human aspect of monitoring from the process of diabetes management. It is also imperative for innovations to address the patient compliance issues associated with current devices, such as injections and needles. Only if these two criteria are met will innovations succeed in the market.
About The Author
Manya Aggarwal is a senior analyst at Decision Resources Group. She has authored several reports on laparoscopic, gynecological, and reprocessed device markets in the endoscopy division of the company. Aggarwal holds a master’s degree in financial economics and a bachelor’s degree in commerce and economics from the University of Toronto.
