DNA Origami Provides Clues About Cancer
By Joel Lindsey
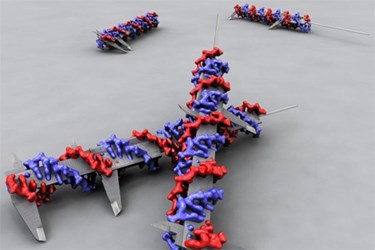
Researchers at Sweden’s Karolinska Institute have used “DNA origami” — a method in which a DNA molecule is carefully shaped into a particular nanostructure — to better understand the role of cell signaling in the spread and treatment of diseases.
In particular, the research team set out to test the function of the EphA2 receptor in the development of several forms of cancer, including breast cancer.
To begin their test, the scientists used DNA building blocks to construct a rod that could be used to conduct precise measurements of the distance between molecules. They then attached ligands — proteins that communicate with other molecules — to various points along the rods. Finally, they placed the entire nanostructure into a solution that contained breast cancer cells.
After observing the response of the cancer cells to the ligands, scientists involved with the project reported that when the ligands were placed close together on the DNA rod, certain receptors in the cancer cells became very active.
The cancer cells also became noticeably less invasive toward other surrounding cells, possibly indicating that these cells, reacting to the closely spaced ligands, could be less likely to metastasize.
“For the very first time, we have been able to prove this hypothesis: the activity of EphA2 is influenced by how closely spaced the ligands are on the surrounding cells,” Björn Högberg, lead researcher at Karolinska’s Department of Neuroscience, said in a press release published recently. “This is an important result in itself, but the point of the study is also that we have developed a method for examining how cells react to nearby cells in a controlled environment, using our custom DNA nano-calipers.”
Researchers said that the study, the results of which have been published in the journal Nature Methods, could be readily applied to a variety of other medical-related applications.
Their use of DNA origami could indicate that cells communicate through a process similar to Braille, in which not only the number of proteins present in a molecule impacts the way it communicates with surrounding particles, but also the physical pattern and proximity of the proteins.
The study could also help improve the ways pharmaceutical drugs are developed, providing additional insight into the ways cells communicate with one another.
“This is a model that can help us learn more about the importance of the spatial organization of proteins in the cell membrane to how cells communicate with each other, something that will hopefully pave the way for a brand new approach to pharmaceuticals in the long term,” said Ana Teixeira, a researcher with Karolinska’s Department of Cell and Molecular Biology. “Today, the function of the pharmaceuticals is often to completely block proteins or receptors, but it is possible that we should rather look at the proteins in the biological context, where the clustering and placement of various proteins are relevant factors for the effect of a drug. This is probably an area where there is important knowledge to obtain, and this is a way of doing it.”
Image Credit: Björn Högberg
