Domain Pharma Introduces Clintrace Upgrade
Clintrace allows companies to unify all adverse event records, regardless of the source, phase, or classification. Through this unification, users gain a complete and accurate picture of a product's safety profile and can respond more effectively and efficiently to regulatory adverse event reporting requirements. By entering and maintaining both pre-and post-market adverse events in one database, Clintrace users can analyze data and track reports more easily throughout a product's entire life cycle.
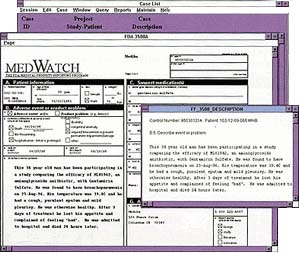
"Clintrace Release 2.8 features a dramatically improved graphical user interface that truly reflects the caliber of our pioneering safety reporting software," said Janet Schorr, product marketing manager of Domain Pharma Corp. "Clintrace's newly developed Case Assignment and Task Manager features accelerate and streamline reporting while ensuring that data is meticulously tracked throughout the system."
New Features
Clintrace Release 2.8 includes a state-of-the-art graphical user interface that color-highlights required entry fields. New "Case Assignment" and "Task Manager" functions allow task tracking and management at each step in the workflow, ensuring that cases are routed correctly through the organization to increase accuracy and provide safeguards against data loss. In addition, Release 2.8 also offers a spell check tool that supports multiple languages, a labeling information reference table, and three levels of online help.
For more information: Kim Bigler, Domain Pharma Corp., 10 Maguire Rd., Suite 110, Lexington, MA 02421. Tel: 781-778-3700. Fax: 781-778-3800.
