6 Essential Steps Of Forensic Medical Device Engineering
By Thom Wyatt, principal engineer, Springboard Pro

Forensic medical device engineering is a high-stakes discipline in which experts investigate and analyze medical devices that have malfunctioned, failed, or caused adverse events. These issues can have far-reaching consequences, from endangering patient safety to affecting legal proceedings and the reputation of medical device manufacturers. Solving these challenging problems requires a robust, systematic approach to identify root causes and implement effective solutions.
Here are six essential steps for resolving forensic medical device engineering problems.
1. Define And Describe The Problem
Establish a clear, evidence-based problem statement to serve as the foundation of the investigation. Gather all information about the problem such as reports, failed devices, manufacturing and quality control data, and user feedback. The 5W2H method can assist the definition (what, when, where, why, how, and how many). Define the criteria for acceptable solutions at the start so that success can be judged objectively. These criteria should be risk-based and realistic; don’t arbitrarily aim for zero failures. For devices on the market, identify the actual and potential impacts on patient safety and public health, and implement a containment strategy to isolate the problem from any users.
2. Replicate The Failure
The problem statement should be demonstrated by inducing failure in representative devices. Depending on the type of failure, non-standard use might be necessary, such as simulating specific environmental conditions, device orientations, etc. Such a benchmark test is important later when testing solutions.
In some cases, the conditions to replicate failure might not be known or the incidence of failure might be extremely rare. In such cases, stress tests can be used to induce failure, such as applying higher forces or elevated temperatures; however, these modifications must be informed by sound scientific logic to avoid producing confounding data.
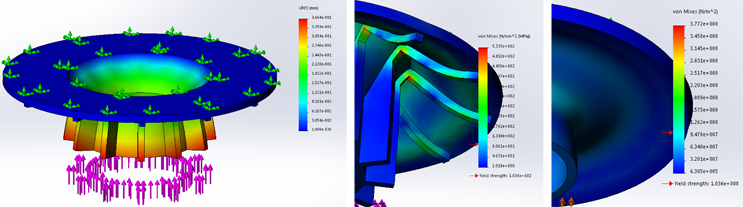
Additionally, advanced simulation tools allow engineers to recreate the behavior of complex systems under different conditions. Finite element analysis (FEA) and computational fluid dynamics (CFD) models built on solid understanding of the underlying physics enable the performance of a device to be evaluated without the need for a complex and time-consuming testing regime.
Figure 1: Hyper elastic FEA model of two components and a liquid silicone rubber.
3. Perform Forensic Analysis
Traditional techniques such as failure mode and effects analysis (FMEA) may not be appropriate because they can cause tunnel vision and be cumbersome. Graphical tools such as functional maps and cause-effect chains are useful to efficiently capture a comprehensive picture of potential root causes. These visual analyses help convey a clear overview of the problem and the interrelationships of potential causes.
Commonly, even seemingly simple failures can have multiple and interacting root causes. The likelihood of potential causes can then be assessed using existing evidence, such as manufacturing data, or by conducting targeted testing on the device, subsystems, or individual components. All evidence must be objective — never rely on unsupported hearsay or opinions!
If relevant, consider using material testing techniques such as spectroscopy, microscopy, and mechanical testing to assess the properties and integrity of various materials used in device construction. This analysis helps identify material defects, degradation mechanisms, and compatibility issues that may compromise the device's performance or longevity.
In many cases, device failures can be attributed to human error or ergonomic issues rather than technical flaws. Employ principles of human factors engineering to evaluate the user interface, interaction design, and ergonomic factors influencing the usability and safety of a device. By conducting usability studies, task analysis, and cognitive walkthroughs, you can identify potential user-related issues to identify design improvements that can enhance user experience and minimize errors.
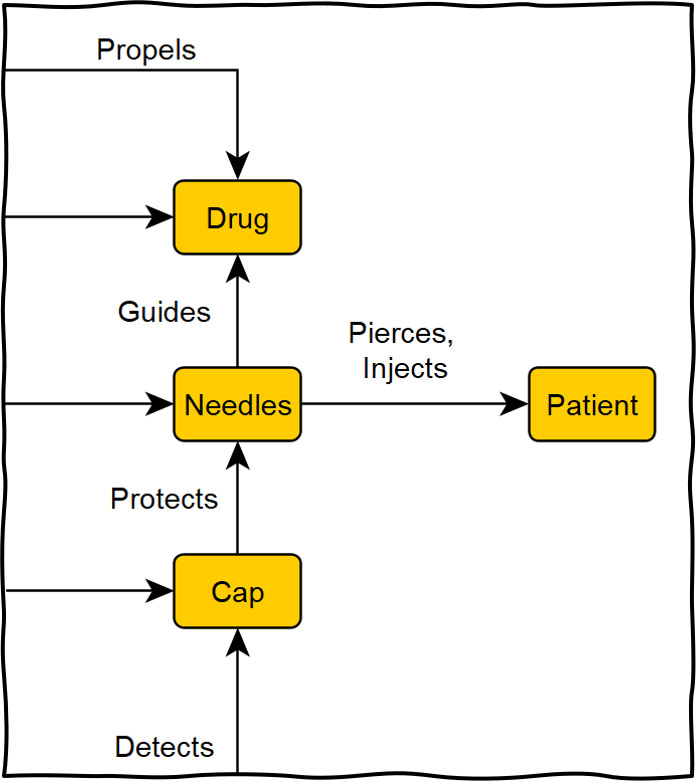
Figure 2: Extract from a functional map for an injection device

Figure 3: Extract from an example cause-effect chain
4. Verify Root Cause
Once the likely root causes of the problem have been identified, the next step is to confirm that they are actually the root causes of the issue. This is best accomplished by attempting to reproduce the failures with and without the suspected root cause. At this stage, you don’t need to focus on detailed solutions but can use simple analogues. For example, if high friction is a suspected cause, tests can be performed with and without plentiful lubrication. This might not be a suitable long-term solution, but it will demonstrate the effect of lowering the friction.
5. Develop Solutions
Once the root causes of the problem have been identified, the next step is to develop and evaluate potential solutions. This may involve redesigning the medical device or components, implementing changes to manufacturing processes, updating quality control procedures, or providing additional training and education. Compare the solutions to the criteria set at the beginning of this process and involve all relevant stakeholders in ranking and selecting the best solutions. Whatever solutions are selected, it is vital to verify that they resolve the root causes of the problem. Repeat the benchmark tests that were used previously to reproduce the failure, to demonstrate that the solution effectively reduces the occurrence of the failure. As with any changes to a medical device, it is important to consider the potential impact of proposed solutions on patient safety, device effectiveness, and regulatory compliance.
6. Implement Preventive Measures
Finally, it's essential to implement preventive measures to minimize the risk of similar problems occurring in the future. This may involve updating design documentation, revising manufacturing protocols, conducting additional testing and validation, and improving post-market surveillance. Communication and collaboration between all stakeholders, including manufacturers, regulatory agencies, healthcare providers, and patients, are crucial to ensuring the effectiveness of preventive measures.
Conclusion
Resolving forensic medical device engineering problems requires a systematic and multidisciplinary approach, involving thorough investigation, forensic analysis, and the development and implementation of effective solutions. By following these six essential steps, medical device manufacturers can identify root causes, mitigate risks, and improve the safety and reliability of their products, ultimately contributing to better patient outcomes and public health.
 About The Author:
About The Author:
Thom Wyatt is a principal engineer at Springboard Pro. He is experienced in the design and development of high-risk medical devices.