FDA Approves Siemens Healthineers More Precise, Lower Radiation CT Scanner
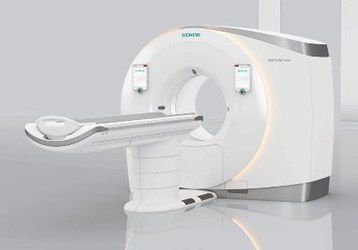
Siemens Healthineers announced the FDA’s clearance of its Somatom Drive computed tomography (CT) system, a dual source scanner indicated for a number of clinical disciplines, including pediatrics, cardiology, and oncology. The system allows for high quality imaging at lower doses of contrast and radiation, less follow-up care, and less need for sedation or other preparation.
Though research has not shown any long-term danger of CT scanning, prolonged and repeated exposure to radiation may increase a patient’s risk of cancer and CT scanning technology is not suitable for all patients, according to the Mayo Clinic. Under certain high-risk circumstances, clinicians have had to rely on imaging alternatives, such as magnetic resonance imaging (MRI) or ultrasound. Siemens’ advanced technology significantly broadens the technology’s range of applications, said the company in a press release.
The Somatom Drive system’s Straton MX Sigma X-ray tubes and Sigma generators allow for more precise targeting and enable high-performance imaging using higher energy levels at lower voltages that are tunable. With adjustable voltages, the system may require less contrast and can be tailored to the specific needs of the patient, as well as reduce unnecessary radiation exposure. For example, less contrast and lower radiation are ideal for critically ill patients, those with reduced kidney function, and small children.
When imaging the chest, the slower speed of traditional CT systems requires patients to hold their breath for portions of the scan or take a beta-blocker medication that slows the heart. These preparations and requirements can limit the technology’s feasibility for children, patients in cardiac distress, or patients in the emergency room. In comparison, the Somatom Drive system can generate high-quality images in between heartbeats, thus requiring less prep time, risk, and inconvenience.
“Siemens Healthineers is proud to introduce the high-performance SOMATOM Drive dual source CT system, which provides our customers with the flexibility to delivery more precise imaging—and potentially further reduce patient dose through the utilization of multiple kV settings,” said Douglas Ryan, VP of CT at Siemens Healthineers North America.
Multiple kV settings allow clinicians to adjust the system for multiple indications. The Dual Energy mode can distinguish between bone and tissue, making it an ideal imaging system for potential spinal injuries or other orthopedic indications.
Siemens recently signed an agreement with Insightec, a medtech specializing in MR-guided focused ultrasound therapy. Together, the two companies plan to develop greater compatibility between Siemens’ MRI systems and Insightec’s Exablatae Neuro system, indicated for the treatment of essential tremors.
Siemens Healthineers is also collaborating with scientists at Case Western Reserve to further develop a technology known as magnetic resonance fingerprinting, which is a new approach to MR imaging that could establish disease biomarkers and make the scans more quantitative, a “paradigm shift” for the technology.
