FDA Approves Siemens "Single Room" CT Platform With Tablet-Based Automated Workflow
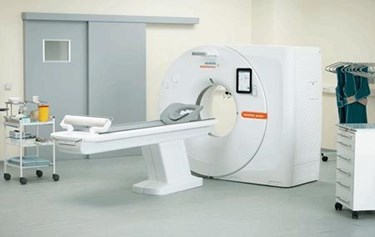
Siemens Healthineers has announced FDA clearance of its Somatom go. CT platform — including the go.Now and go.Up models — which allows technicians to control the equipment via tablet in the same room as the patient. The design, unveiled at the Annual Meeting of the Radiological Society of North America in November, was based on input from over 500 radiologists who participated in workshops hosted by Siemens.
Traditional CT scanning systems can take up two or three rooms, with the scanner in one room and associated computer hardware in a separate “control room.” The highly complex system requires extensive training and expertise on the part of technicians to produce consistent, high-quality imaging. The Somatom go. CT platform seeks to automate the CT business, improving its accessibility with “workflow and usability innovations that improve efficiency independent of the individual user’s level of experience.”
The standardized and automated workflows facilitated by the tablet, said Siemens in a press release, improve clinical results with a “lower total cost of ownership.” The company describes the go.Now model as “low cost, but clinically robust,” providing 32 slices. In comparison, the go.Up model offers 64 slices, which is particularly useful in providing quick scans of lungs without requiring patients to hold their breath for uncomfortable periods of time.
The single-room operation of the go. CT platform also provides advantages in environments with limited space availability, and the setup provides a “shielded niche” to protect radiology staff from radiation. In addition to reducing installation costs, the system aims to improve patient experience — especially for small children — allowing them to undergo a scan without being left alone in the room.
“If a patient feels that someone is close by, it is perceived as better care,” said Matthias May, a professor at the Institute of Radiology as the University Hospital in Erlangen (UHE), in a press release in November. Michael Uder, director of the Institute of Radiology at UHE, added that workloads are increasing just as budgets are shrinking, and radiologists have a need for improved workflow efficiency.
Both models in the go. CT platform come with features previously only offered with the company’s most advanced CT scanning systems, including the Stellar detector, which offers ultra-thin slices and high spatial resolution, as well as additional innovations that potentially reduce radiation used by the scanner and require less maintenance.
According to Andre Hartung, director of Computed Tomography at Siemens, researchers at Siemens spoke to over 500 radiologists and technicians to design a system that that “offers high quality standards and responds to the current needs for efficient workflows, and to clinical and financial requirements” in both established and emerging markets.
Earlier this year, the FDA approved a “budget friendly” MR system developed by Siemens for small to medium-sized hospitals. Likewise, the system aims to improve radiology workflows and respond to mounting concerns over reimbursement.
Siemens CEO Joe Kaeser recently put an end to rumors that the company would completely divest Healthineers, saying that Siemens intends to retain a majority stake, because healthcare could present a larger growth opportunity than the company’s industrial and energy sectors.
