FDA Clarifies UDI Form And Presentation In Device Labeling
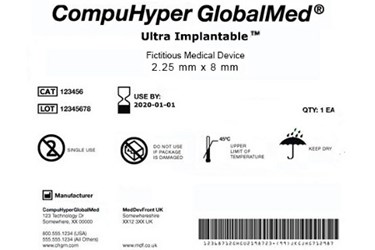
The FDA has drafted a guidance clarifying the form and presentation of the unique device identifier (UDI) on medical device manufacturers’ packaging and products to encourage greater compliance with the FDA’s UDI Rule. According to the FDA’s explanation, UDIs must be presented in “easily readable” plaintext and Automatic Identification and Data Capture (AIDC) forms, and elements of the UDI must be presented in a consistent order.
The UDI Final Rule — published in 2013 — requires all qualifying medical devices to bear an identifying tracking number on labeling, product packaging, and the device itself if it is to be used more than once — unless a suitable alternative is authorized by the agency. All labeling must be submitted to the Global Unique Device Identification Database (GUDID).
The FDA began rolling out this program in 2014 and the rollout is expected to continue through 2020, according to the Regulatory Affairs Professionals Society (RAPS). Class III devices and implantable and life-supporting technologies already are subject to the rule, and Class II devices will be expected to comply with this rule no later than the end of this year.
In its most recent UDI guidance, the FDA stipulates that both the AIDC and plaintext labels be “easily readable” and placed within close proximity to each other. If there are space limitations, both the automated and plaintext labels may be split into multiple lines or barcodes, but each must display the device identifier (DI) number before the production identifier (PI). If the manufacturer wishes, they may include more than one AIDC label to comply with multiple types of capture technology.
The guidance allows “FDA-accredited issuing agencies” that provide the UDI numbers to include additional information and numbers outside of the DI and PI, but the agency stated it will not recognize any of the additional data as part of the UDI. Further clarification of the specific ordering of data — both UDI and non-UDI — is also outlined in the guidance.
“The UDI elements should be able to be readily distinguishable and captured separately from any non-UDI elements that may be represented in the UDI carrier,” wrote the FDA, adding that “data delimiters” should be included to allow users to distinguish DI from PI numbers. Approved data delimiters were outlined in a separate document.
The FDA and CMS currently are encouraging legislators to require UDI numbers on insurance forms, including documents used by Medicare. Though adding the information to existing forms will cost time and money, proponents of the legislation — including Elizabeth Warren (D-Mass.) and Charles Grassley (R- Iowa) — claim the added information could save CMS billions of dollars long-term and improve patient safety.
Though implementing UDIs into the manufacturing system has proven a complicated and challenging process, recent developments within the E.U. with its Medical Device Regulations (MDR) show that the continent is moving steadily toward adoption of the program. In a recent column, Steve Cottrell, president of Maetrics, and Madris Tomes, CEO and founder of Device Events, told MDO that the U.S. has provided template for the process and companies that learn from the U.S. will “gain competitive advantage.”
Device manufactures and other healthcare industry stakeholders will be able to comment on the current guidance for the next 60 days.
