FDA Cleared Icare HOME, An Innovative Device Poised To Revolutionize IOP Self-Monitoring
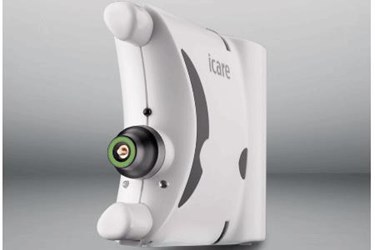
Icare USA, a subsidiary of Icare Finland, the original developer and global leader in handheld tonometry, announces that the Icare HOME tonometer has been cleared by the FDA and is now available for use in the United States.
The Icare HOME device, which received CE Marking in 2014, has quickly become an essential tool in Europe. Eye care professionals have come to rely on the added clinical data it provides of how their patients’ IOP fluctuates throughout the day. Thanks to this recent clearance by the FDA, doctors in the United States can also now benefit from the ability to monitor patients with more regularity and confidence.
“The fact that we can put a tonometer of this caliber in the hands of patients is truly unprecedented,” says John Floyd, President and CEO of Icare USA. “The Icare HOME makes doctors aware of dangerous spikes that they may never have known about otherwise. The impact and level of care that this provides cannot be overstated.”
The Icare HOME uses the same patented rebound technology that eye care professionals have enjoyed and trusted in other Icare tonometers, such as the ic100 and the TA01i. The Icare HOME unit requires no specialized skills for patient use.
Several features make the Icare HOME easy for patients to use. For instance, the unit’s built-in Icare EyeSmart technology performs automatic OD/OS recognition. Positioning is also easy thanks to Icare EasyPos, which uses red and green light signals to help patients correctly position the tonometer. The Icare HOME also features Icare AMS, an automated measuring sequence that can take either a single measurement or a series of six measurements with one touch of a button.
The Icare HOME involves no puff of air and requires no drops. In the hands of patients, it is quick, effortless and effective. For eye care professionals, the Icare HOME is designed to become a staple of care in the management of millions of patients who could benefit from additional IOP monitoring.
About Icare USA
Icare USA is a subsidiary of Icare Finland, the original developer and global leader in handheld tonometry. The advanced Icare product line offers reliable, high precision, reproducible accuracy in measuring intraocular pressure in any setting. The company holds more than 30 patents and patent applications as well as a variety of widely-used Icare tonometers that are designed for both experienced and inexperienced users.
Source: Icare USA
