FDA Clears XSTAT Hemostatic Dressing For Civilian Use
By Jof Enriquez,
Follow me on Twitter @jofenriq
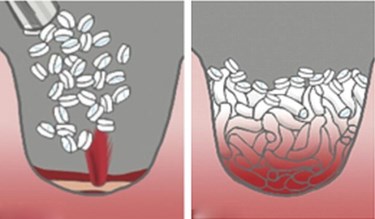
The U.S. Food and Drug Administration (FDA) has given startup RevMedx 510(k) clearance to market the XSTAT 30 wound dressing for civilian use. The agency deemed the product equivalent to the XSTAT dressing, which was FDA approved for use by U.S. military personnel, through the de novo classification process, in April 2014.
The first-of-its-kind hemostatic device comes in syringe-style applicators containing 92 compressed, cellulose sponges with an absorbent coating, according to an FDA press release. The small sponges are injected via the applicator into a wound cavity, where they expand and swell up to 10 times their size to fill up the wound space, absorb blood, exert hemostatic pressure, and create a temporary physical barrier to blood flow. The dressing can be used for up to four hours, giving emergency first responders enough time to bring the wounded patient to a trauma facility.
According to the United States Army Institute of Surgical Research, 30 to 40 percent of civilian deaths by traumatic injury are caused by blood loss. Of these deaths, 33 to 56 percent occur before the patient reaches the hospital.
“When a product is developed for use in the battlefield, it is generally intended to work in a worst-case scenario where advanced care might not be immediately available,” William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health (CDRH), said in the release. “It is exciting to see this technology transition to help civilian first responders control some severe, life-threatening bleeding while on the trauma scene.”
Previously cleared for military use only, XSTAT 30 is now approved for adult and adolescent civilian patients at high risk for immediate, life-threatening, and severe hemorrhagic shock, and for non-compressible junctional wounds not amenable to tourniquet application, such as in the groin or axilla (armpit), according to FDA. The product is contraindicated for use in certain parts of the chest, abdomen, pelvis or tissue above the collarbone.
An article published in New York Mag says “the impetus for creating XSTAT came from the U.S. military, which contacted RevMedx in 2008 during the Iraq War, when battlefield medics needed a better way to stop soldiers' bleeding until they could be brought to a hospital."
U.S. military researchers are exploring new device technology to speed combat triage, including attaching microcurrent matrices to wound dressings to hasten healing, and making wearables that detect mild blood loss before it becomes clinically apparent.
