FDA Issues Final Rule On Symbols In Medical Device Labeling
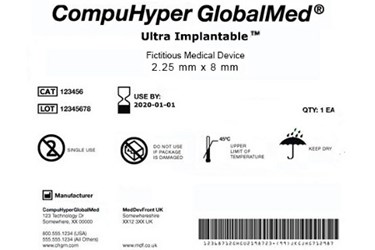
The FDA has released a final rule governing the use of symbols on device labeling that will allow manufacturers to use standalone symbols — which have been in use overseas for years — without adjacent explanatory text, as long as certain conditions are met. The rule is expected to simplify the labeling process for devices marketed internationally and save manufacturers up to $25.5 million.
The agency first introduced a proposed rule in 2013, suggesting that the agency allow symbols in labeling for devices and in vitro diagnostics, but only if they were developed by a standards development organization (SDO) recognized by the FDA and accompanied by a symbol glossary to be included in the literature sold with the device.
In solicited comments, the Advanced Medical Technology Association (AdvaMed) argued that requiring the explanatory text negated any advantage a manufacturer might have in using the symbols, as that text would still have to be printed and translated in several languages in accordance with international market standards. AdvaMed proposed the FDA drop the glossary requirement and issue a rule in accordance with the EU’s Medical Device Directive.
“A goal of adopting the use of internationally recognized symbols without adjacent explanatory text is to reduce the cost of maintaining multiple inventories of the same product and to avoid increasing packaging and labeling size to accommodate the text and symbol while at the same time providing clear information,” AdvaMed wrote. “However, the current proposed rule does not help to reduce the cost of the device.”
Despite these criticisms, the final rule does require a glossary for symbols included and only eliminates the requirement that explanatory text be adjacent to the symbol. However, the labeling may include an external link to a glossary located online. According to the Regulatory Affairs Professionals Society (RAPS), the most significant difference between the proposed rule and the final rule is that the agency has decided to allow symbols developed by SDOs not recognized by the FDA.
According to FDA, manufacturers may make a determination about included symbols independent of the FDA-recognized standards if “the symbol is likely to be read and understood by the ordinary individual under customary conditions of purchase and use.”
The final rule also clarifies the agency’s definition of SDO, outlined required elements of the symbols glossary, and formally allowed for the use of Rx Only for devices only available with a prescription. In a separate notice, the agency published a revised list of symbols it currently recognizes.
“Permitting use of standalone symbols is an important step toward global harmonization of device labeling. We commend the FDA for finalizing this important rule,” said law experts from Hyman, Phelps and McNamara in a post on FDA Law Blog.
According to the FDA, the ruling is optional and device manufacturers may continue publishing their labeling with the adjacent explanatory text, if they choose. The agency estimated that the rulings’ cost savings would “roughly range between $7.9 million and $25.5 million.”
