Foundational Human Factors Engineering Concepts For The Design Of Combination Products
By Renée Bailey, senior manager, human factors engineering, BlackHägen Design

If you are involved in designing and developing devices for combination drug and delivery products, you would likely agree that there are many complexities inherent in the development of these products. With growing patient and physician preference for minimally invasive procedures and consistent-dosing treatment alternatives, the demand for these products is forecast to increase over the next several years. According to Grandview Research, the global drug-device combination products market size was valued at $127.8 billion in 2022 and is expected to grow at a compound annual growth rate of 8.9% from 2023 to 2030.
As drug and device development advances, global regulatory requirements also continuously evolve and add their own layer of complexity. In September 2023, the U.S. FDA finalized its guidance regarding the application of human factors engineering (HFE), also called usability engineering (UE), to combination products. The finalized guidance further clarified and cemented the FDA’s expectations for applying HFE throughout the product design and development process and the importance of addressing use-related risks. So, how can companies keep pace with the demand in this highly competitive market while meeting or exceeding regulatory expectations of applying HFE?
Applicable FDA Guidance At A Glance
It’s important to understand all the applicable regulatory guidelines and expectations before initiating the design and development of combination products. Globally, there is a regulatory expectation that HFE is applied to ensure the product is safe and effective in the hands of the intended end user(s) in their environments of use. With the recent finalization of the combination product guidance, the FDA now has three final HFE and design guidance documents that are key references for the design and development of combination products:
- Applying Human Factors and Usability Engineering to Medical Devices, February 2016 (Note: The FDA also recognizes IEC 62366 for the application of HFE/UE during product design and development.)
- Application of Human Factors Engineering Principles for Combination Products: Questions and Answers, September 2023
- Safety Considerations for Product Design to Minimize Medication Errors, April 2016
While these are the core FDA guidance documents to consider, other guidance documents could also apply or be informative. For example, one guidance document details design considerations for pen, jet, and other injection drug delivery devices, and another goes more in-depth about emergency-use injectors. (For a complete list of guidance documents, including a few device-specific documents, visit the FDA website.)
Product development teams should conduct research to reference any applicable guidance documents or work with a regulatory affairs stakeholder to understand the regulatory pathway and any additional requirements for the combination product. In the U.S., various pathways to regulatory approval exist depending on the product (e.g., new drug application, abbreviated new-drug application, etc.). Working with a regulatory affairs stakeholder is essential to identify the necessary HFE requirements and ensure the appropriate or required HFE activities are conducted accordingly from the onset.
Triad Of HFE Inputs Related To Product Design & Development
The complexity of combination products stems from the need to ensure the whole product user interface (drug, device, packaging, labeling, instructions for use, etc.) is safe for all potential users and their use environments, minimizes the potential for medication errors, and ultimately delivers the intended therapy to the patient. To accomplish this, the FDA outlines a triad of HFE inputs instrumental in supporting iterative product design (see Figure 1):
- Analysis of all potential users and all potential use environments
- Risk management, specifically as it pertains to use-related risk analysis
- Iterative human factors evaluations
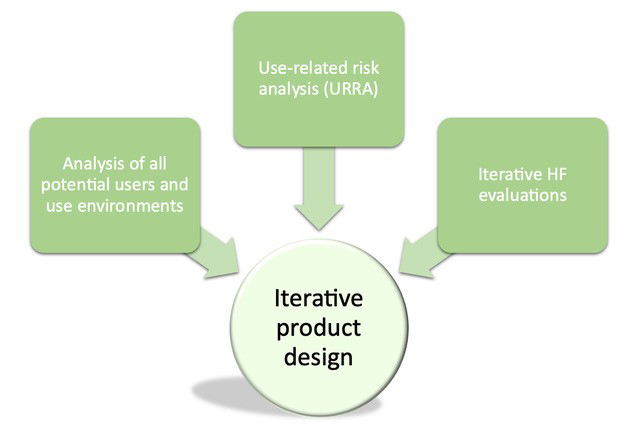
Figure 1: The triad of HFE inputs to product design & development. © 2024 BlackHägen Design.
Integrating these aspects of HFE is the gold standard in combination product development, and the risks are great when they are overlooked or not completed rigorously. And not only do these inputs inform the product design, but they also inform each other, for example:
- Analysis of all potential users and their use environments: Early user research identifies all potential users, provides detailed descriptions of their capabilities and limitations, and the characteristics of their use environments. This analysis not only provides crucial information for exploring a new product design and early concepts, but it also contributes to the use-related risk analysis (URRA) and HFE evaluations. To develop the URRA, comprehensive analysis of users and their environments is required to identify tasks, subtasks. and potential use errors associated with task performance. Additionally, the analysis defines who should participate in HFE evaluations and the setting in which the evaluation(s) should take place.
- URRA: Leveraging the user and use environment analysis, the URRA defines all use cases, tasks, and subtasks related to the user interface, potential use errors, the potential harm to the patient or user that results from potential use errors, and the severity of that harm. Additionally, it describes which aspect(s) of the user interface or product design is intended to minimize the identified risks (i.e., risk control measures). When developing HFE strategies and planning HFE evaluations, the URRA is used to define the critical tasks associated with device use.
- Iterative HFE evaluations: HFE evaluations involve testing the user interface with representative end users in a representative use environment(s) to ensure the device can be used safely and effectively. The participants and settings of evaluations are selected based on the user and environment profiles developed in early HFE activities such as contextual inquiry or ethnographic research. The overarching HFE plan and individual HFE evaluations are based on the product’s user interface design and corresponding tasks and subtasks that need to be evaluated from the URRA. HFE evaluations are conducted to gather data on task performance, use issues encountered by participants, and whether the implemented risk control measures were effective. Where risk control measures are ineffective, the product design is iterated, if possible, with new or additional risk control measures and, in turn, the URRA is updated to reflect these changes.
As you can see, these HFE inputs are inextricably linked to product design and development activities. And at the heart of them is the URRA. Yet, often, the URRA is not used effectively by product development teams, leading to missed opportunities to track design-related risks that serve to improve the design and safety of the user interface. This, in turn, can lead to costly delays after regulatory submission and added costs to conduct or repeat additional HF evaluations to meet the FDA requirements. In my next article, I’ll focus on URRA for combination products.
About The Author:

Renée Bailey is the senior manager of human factors engineering at BlackHägen Design. With nine years of experience in human factors engineering and 26 years as a Certified Instructional Technologist (CIT) and performance improvement consultant, she has worked with large-scale programs in Fortune 100/500 companies and various regulated industries, including medical device manufacturing, pharmaceutical, utilities (oil & gas, electric), finance, and food safety. Bailey has experience applying regulatory-focused human factors best practices to leading project teams, project strategies, use-related risk analysis, threshold analyses, and human factors study execution for pre- and post-market programs. Significant experience includes projects related to drug-delivery combination products.
