How Is Medtech Design Evolving — & Are You Keeping Up?
By Philip Remedios, BlackHägen Design

So much has happened in every society across the globe since the COVID-19 pandemic that lifestyles have been displaced and employer attitudes toward a work-from-home concept have shifted to the positive, supported by modern IoT tech and slick online connectivity tools. A decentralized workplace theme is emerging that threatens historic norms, including how people wish to manage their health. The idealism behind home-based or distance care constructs is not new, but in the recent past it has been largely driven by the desire to reduce operating costs rather than to enable patient-driven desires. Recent COVID-based medical practices have encouraged out-of-hospital consultation and remote diagnostics to reduce viral transmission and hospital burden and enable a workflow that supports multiple advantages – less clinical overhead, higher efficiencies, faster transactions, and greater convenience for all parties.
Couple the events of the last 12 months with the last decade’s trend toward overhead reduction through distance care, and the combination is driving more rapid change to the medical device environment than ever before. This article takes a glimpse at the recent evolution in medical device design and speculates how it might evolve in a post-COVID world.
Evolution Of Design Innovation
Twenty years ago, innovation in the medical device space was still strongly associated with advancing capability, accuracy, and convenience. Capital equipment became more complex and expensive with every iteration, along with prepackaged single-use disposables that even included integrated electronics. Budget-conscious emerging markets were the first to push back from this costly new trend, along with European nations that were trying to minimize product waste by maximizing recycling programs. Designers were forced to reconsider the concept of 100% disposable designs to enable business expansion to cost-sensitive markets.
I first noticed a change in new design requirements about a decade back. Initially the push was being driven by emerging markets clamoring for lower-priced alternatives to first-world devices and procedures, including limiting “bells and whistles” to only critical features. But untenable healthcare costs, primarily in the U.S., began to support the need for change. A trend was setting in to launch new low-cost versions of legacy devices in these emerging international markets before bringing them back onshore, sometimes deploying a half-generation iteration to optimize them for domestic market needs.
Innovative design was increasingly being defined as maintaining and sometimes reducing “cutting-edge” functionality but at substantially lower per-case cost. Requests to reduce operating overheads by 30, 50, and even 80% have become the norm, requiring wholesale reconsideration of not only the device itself, but of the deployed technology, materials, procedure, reusability, and, therefore, sterilization techniques. As optimized usability has become a necessary ingredient of good design practices, use-safety and ease-of-use attributes also have to be included without cost impact.
Other important cost-cutting measures include operational efficiency and maximized procedural success. When one considers that operating room time can cost $100/minute, reducing the time it takes to perform a procedure by 20% or 30% can realize considerable savings, not to mention an institution can increase profits by performing more cases per day. Furthermore, if a design can effectively increase the chance of procedural success by limiting use-error and therefore also revisional action (which is usually not reimbursable), the hospital benefits with further operational cost savings.
Prioritization Of Cost & Risk Reduction
In a concerted effort to reduce overall costs, device end users are evolving as well. Lower-paid clinical fellows, nurses, and med techs now routinely set up and operate devices that were once managed by the physician or surgeon. This means user interfaces must often be simpler and more intuitive to be operated by inexperienced and/or lesser-trained staff. Solutions often include developing varying levels of interface operability depending on the user set and locking the limits on procedural protocols, enabling med techs to access everyday setup and operations controls while nurses supervise and monitor only critical parameters during their shifts to minimize the mental burden.
Changing user requirements also influences the design of recent devices. For capital equipment, mobility is necessary when an intermittently used device needs to be shared among operating rooms, wards, and other points of care (POC) in order to reduce CapEx. Devices that remain in place should be accommodated on shared storage systems or attached to off-the-floor gantries, as floor space is often at a premium and cable trip hazards are an everyday problem.
Devices intended for shelf- or rack-mounting require the user interface to be entirely located on the front face. This may not seem like a design challenge, but consider those devices that require significant single-use coupling, such as peristaltic pumps, filters, connections, and sensors, which leave little room for a decent sized graphical display and hard keys. One advantage of modern technology is that standardized software protocols now allow many devices to display data on a shared monitor, which alleviates some of the user experience (UX) challenges. Careful study and consideration of current and trending use cases will enable the design team to conceptualize the most appropriate system architecture to meet real-world needs for the useful life of the device.
The Patient As User
The ongoing trend to enable the patient to be more involved in their healthcare has resulted in a proliferation of new devices intended for home and wearable use. Initially, these devices were diagnostic in nature, helping providers monitor the vital signs of critically ill patients on a daily basis, but digital technology has launched an interconnected society where secure data can be gathered, transmitted, and assessed with relative ease. Wearable pumps, drug delivery patches, and countertop devices are now commonplace, with new reimbursable codes supporting the trend, and the industry is capitalizing on this new interoperable paradigm to further advance healthcare. From a designer’s perspective, these patient-operated devices must be considered as consumer products and should provide the same slick aesthetics and ease of use as a smartphone or kitchen appliance.
The following patented concept by BlackHägen Design illustrates how a global design platform can be adapted to suit a variety of market- and context-driven installation and UX requirements. The base housing is designed around the electronic controller and power system, with modular side caps and a front display module so that the platform can be factory configured to suit varying market drivers, such as cost, functionality, feature sets, and environmental workflow:
 |
Minimal graphical user interface (GUI) configuration is required in a shelf- or rack-mount application. Note that all interfaces are accessible from the front, including the side-located but front-accessed and visualized peristaltic pump, with hard keys and connectors mounted to the GUI surface. |
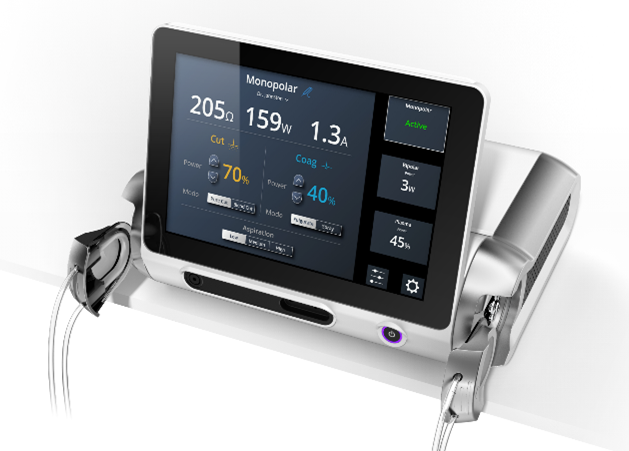 |
This shows an intermediate GUI configuration for table or roll-stand mount. The display may also rotate to optimize viewing angle depending on mounting option. Hard keys and connectors may be mounted below the GUI, with dual peristaltic pumps or alternative interfaces on either end. |
 |
A maximized GUI configuration provides a full-width display required for graphics-intensive applications such as ventilators and dialysis machines. These devices may be preferably roll-stand or arm-mounted for total mobility due to the criticality of the display data and the need for system controls to be highly visible and close at hand. |
Maximizing cost-reducing innovation requires understanding the entire device daily workflow in order to identify areas of opportunity. For example, maintenance and handling requirements also affect overhead costs. Considering features such as storage space efficiency can enhance the marketable value of a new design. In the depiction below, the roll-stand architecture can be designed to nest within adjacent devices, substantially reducing space requirements within a ward or central depot. Interlocking devices can be ganged together for ease of ambulation/portability while also facilitating overnight gang recharging of onboard batteries:
![]()
Durable Versus Disposable Versus Hybrid Instrument Design Strategies
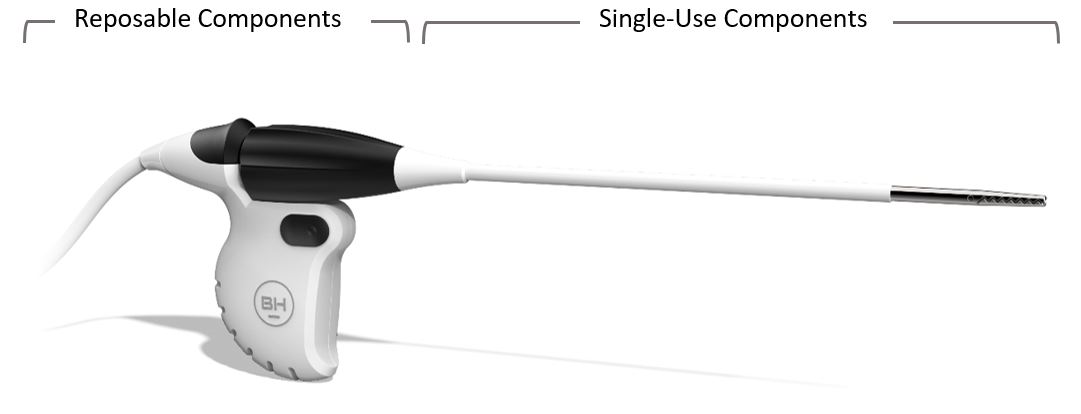
Instruments and handheld devices are migrating away from 100% disposable strategies. By reposing non-wearing, high-cost portions of the device (re-sterilization requirements and costs must of course be added to the design requirements and per-case cost), the sub-cost (the cost of that sub-assembly) can be amortized over hundreds of cases, vastly reducing the adjusted unit cost. Electronics, motors, sensors, and optics that may be embedded in these subassemblies must withstand the selected re-sterilization protocol, which may include high heat, moisture, gas flow, aeration, and material durability. In some markets, it may be a requirement that reposable parts are able to be sterilized using hospital-operated systems, which in many cases is steam-based. The FDA has published a useful guidance document for designing devices for reuse: (https://www.fda.gov/media/80265/download).
Disposable components are now highly scrutinized for their single-use functions and rightfully still include mechanical parts such as needles, cannulas, cutting blades, springs, wearable electrodes, plus part geometries and materials that are difficult to re-sterilize. All other components should be considered for durable design, resulting in “hybrid” devices that must be easy to partition and (dis)assemble reposable parts for cleaning and reuse. The longer the mean-time-before-failure (MTBF), the cheaper the resulting amortized per-case cost of the device.
Concurrent Product Development Strategy
Increased technical complexity usually means increased project risk, invariably lengthening the time-to-market launch of the new device. When the end goal is to become or maintain market leader status for capability and technology, a tried-and-true marketing strategy is to determine the requirements for a minimally viable product (MVP) and develop this as the Gen 1 device. However, the underlying design architecture of the MVP is intentionally created to provide a robust and upgradeable platform to spawn a series of upgraded versions over its life, either for the same or for other markets. In the case of complex devices intended for a long service life, a modular approach can also be integrated that anticipates component(s) obsolescence (such as displays, controllers, data storage, and power systems) and enables localized swap-outs for newer, faster, lower-cost replacements.
A real-world example of how this “pipeline approach” can be deployed was to swiftly develop and launch a low-cost MVP Gen1 device for a targeted international market (China and India in this case) while concurrently continuing development of U.S./EU market modular upgrades to the platform. Subsequent and risker iterations of the device can then be launched into more lucrative first-world markets at higher, sustainable cost points. Another benefit of a staged global release provides actual operational performance, reliability, and clinical data from the initial MVP offering and the ability to optimize the Gen2++ devices before their respective launches. A bonus to this strategy is that the competition is faced with a moving target over time, which is much more difficult to leapfrog.
Summary
The worldwide medical device industry has become incredibly sensitized to cost savings, including CapEx and per-unit costs, as well as achieving maximum operational efficiencies, all while minimizing cost-inducing user errors. The modern innovation team needs to be far more pragmatic in balancing real-market drivers with device technology, which requires a clear knowledge of the competitive landscape, cost limits, and market penetration strategies. Design engineers must adopt macroscopic visibility to these project goals to ensure realization of the project mission. The traditional desire to maximize absolute capability can no longer be the overriding mantra, and more than ever stakeholders are required to scrutinize value-added features and functions against cost creep and project risk.
Finally, it should be noted that despite the unanimous call for cost control, efforts to advance medical technology will always continue because functional capability and positive patient outcomes will always differentiate a company from its competition. But in a maturing and intensely competitive industry, design innovators must capably wear many hats in order to understand and balance the true value proposition in tomorrow’s medical device world.
About The Author:
 Philip Remedios is principal and design director at BlackHägen Design, an R&D consultancy focused on medical device innovation. With a combined design and engineering background, Remedios has spent most of his 35-year career in executive consulting roles, developing project plans and managing integrated technical teams, schedules, and budgets. He has a Bachelor of Science in industrial design/transportation from the Art Center College of Design in California and is a named inventor on 34 patents to date.
Philip Remedios is principal and design director at BlackHägen Design, an R&D consultancy focused on medical device innovation. With a combined design and engineering background, Remedios has spent most of his 35-year career in executive consulting roles, developing project plans and managing integrated technical teams, schedules, and budgets. He has a Bachelor of Science in industrial design/transportation from the Art Center College of Design in California and is a named inventor on 34 patents to date.

