How To Design Next-Generation Devices For Specific Patient Populations
By Shahid Shah, president & CEO, Netspective Communications
Follow me on Twitter @ShahidNShah

Digital health and Big Data are looking to transform the world of medicine through software and next-generation medical devices. In the past, we were able to segment device designs into easy to understand categories like consumer-centric, ambulatory (outpatient), and acute (inpatient). This was because we knew where our devices would be used and who would use them – either the patient directly or, more commonly, a healthcare provider (HCP) on behalf of a patient or an HCP using it for diagnostics on a patient. As we move from fee-for-service to value-based reimbursement and outcomes-driven care models like accountable care organizations (ACOs), it will be increasingly important to design devices not just based on where they will be used, but on what kind of patient population we will be targeting.
Creating patient tiers is never easy, but we can at least start to consider them falling into four general categories:
- Those who are well and we want to help prevent from getting diseases
- Those who are at risk for getting certain diseases
- Those who need help managing a chronic condition they already have
- Those who need acute treatment
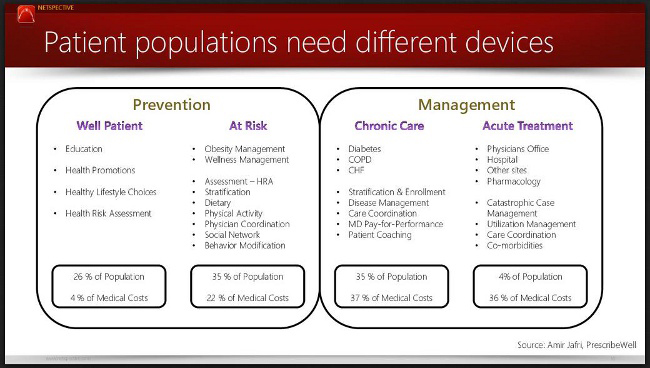
The most important consideration in our medical device designs, especially when considering patient populations, is whether we are targeting a high number of patients or a high cost. The most successful designs will, of course, target the largest number of patients that cost the most to treat. From the figure above, that is most likely patients in the at-risk and chronic care segments.
Once you have identified which patient tier or segment you want to target, it is important to keep your device design generic, but also to focus on specific specialties (e.g., cardiology) or disease conditions (e.g., obesity vs. diabetes). In the past, device designs were based on specialization inside the design itself. In the future, you will need to make sure the design itself is generalized but that the configuration of a particular version of the device, if possible, is specific to a specialty or disease condition. Your next-generation medical device design must be based on a product line architecture (PLA) with off-the-shelf components that will give you the most flexibility to change the design as new payment models and patient tiers are encountered. The way you put the components together and the different product lines you define need to align to the patient tiers outlined above.
Future device designs will have to migrate to a PLA with significant flexibility because the way medical devices will be purchased in the future will be based on different reimbursement models than they are today. Instead of fees-for-services, we are moving to value-based reimbursement, and traditional ways of paying for our devices will change. Here’s how:
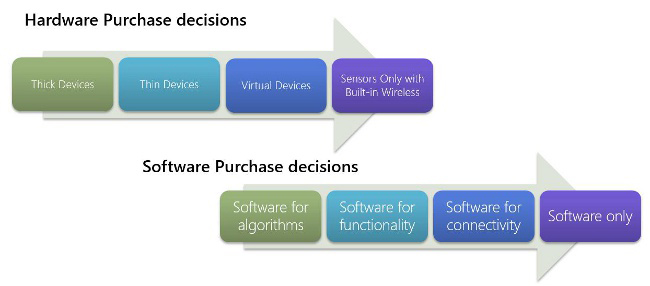
Most medical technology used to be thick devices with all custom components, including database, operating system, embedded utilities, etc. Over time, that has changing to thinner devices and virtual devices (e.g. mobile phone based) to more sensor-only devices. Most hardware in the future will not be sold as a single unit but as separate sensors that will be integrated into next-generation devices.
The software side is changing in a similar way. We used to build software solely to support analog- to-digital conversion or basic algorithms to support thick devices. That changed over time to include software to enhance functionality, and now it is focused on adding more and more connectivity into our devices. The future of medical devices is primarily in software – couple that with the focus on sensor integration and you can imagine future devices being mostly software with no custom hardware at all.
To close, I would like to make some specific suggestions about how to use a PLA and sensors/software to focus on specific patient populations. If you are targeting the well patient, you are focused on low cost and high volume and without physician (or care provider) intermediation. This means that you should run your software on one or more mobile app platforms (probably a native iOS, Android, or Windows platform). Mobile apps can usually accommodate up to Medical Device Data System (MDDS) or Class I FDA regulations but often don’t need it.
If you are targeting at-risk patients, you will want to create a design that can be discovered and used by patients but potentially be “prescribed” (or suggested) by a physician who will be tracking information output from the device in an enterprise system, like an electronic health record (EHR).
If you are targeting the chronic care patient tier, you will want to switch your model to primarily being “prescribed” to a patient by a care provider or institution. You should also make sure that your designs are very enterprise friendly, with features such as data integration with EHRs, storing utilization data to prove effectiveness, and creating claims or other billing functionality. The chronic care devices need to be used by patients but monitored and managed by care providers.
The final patient tier, acute treatment, is of course the one that we are most accustomed to today in the medical device industry. These devices generally are designed in the same – usually expensive – way, but future acute care devices need to also incorporate functionality that will help institutions understand how to more effectively manage, maintain, and deploy larger numbers.
In future articles in this series, we will dive deeper into device designs at the various tiers.
Shahid Shah is a population health, care coordination, and patient engagement coach and an award-winning medical device hardware / software design mentor with 25 years of technology strategy and engineering experience. You can reach him via Twitter or email.
