How To Make The FDA Happy: 7 Medtech Design Pro Tips For Combination Products
By Tom KraMer, Kablooe Design

The world of medical technology is ever evolving, and one significant innovation that's been gaining momentum is combination products. These remarkable medtech products seamlessly integrate both medical devices and pharmaceutical components into a single cohesive solution.
However, as the industry evolves, so do the regulatory requirements. Let’s dive into the unique landscape of combination devices and explore seven pro design tips to not only meet the FDA's stringent standards but exceed them.
Conducting Usability Studies
The FDA likes to see studies — and for good reason. They want to know that your combination device is both safe and effective, and this can only be demonstrated through rigorous usability studies. Make sure your team has the expertise (or partner) necessary to carry out these studies effectively.
Pro Tip #1: Know Your Goal
What are you trying to learn? You can start by gathering as much information about the combination product as you can. Then, once you know your goal, you can develop a study plan that is both efficient and informative.
Pro Tip #2: Know the Regulatory Deliverables
These can include everything from an IDE application to a 510(k) submission. According to FDA guidance, there are five major pre-validation items and two major post-validation items that need to be included in any study.
Pre-Validation Items:
- updated risk analysis from resulting design changes
- summary of formative study results/analysis
- documented design changes and rationale
- a draft of the summative study protocol
- labeling and IFU to test in the summative study
Post-Validation Items:
- summative study (validation) protocol and summary
- summary of total human factors effort
When it comes to combination devices, we have to think a bit deeper about how we will evaluate the device for usability testing. In the FDA's new guidance document, they pay special attention to use errors that might:
- Impact dosing (e.g., an overdose, underdose, or missed dose), including those that may lead to a lack of treatment response;
- Impact administration of the product (e.g., the wrong site of administration, improper preparation of the drug/biologic before administration); or
- Have the potential to result in harm (e.g., physical injury, adverse events, events that may need patient monitoring to confirm no harm, or events that may lead to hospitalization).
Making sure we understand what the users are capable of in these areas, what they are likely to do (and not do), and how we can improve our designs to make it easier for them are all important things for us to consider when designing the features and functions of the combination device.
Pro Tip #3: Conduct Your Summative Studies BEFORE Your Large Clinicals
This is more of a general guideline than a hard rule, as even the FDA acknowledges that there are exceptions to this order.
Design Controls
All combination products must undergo a rigorous design control process, which helps catch and correct potential problems, ensuring your product is safe and effective. The FDA has outlined specific design control requirements in Section 820.30 of their Quality System Regulation (QSR), and it’s important to adhere to these if you want your product to get approval. Think of this as a sort of guideline for all your development activities leading up to production.
Pro Tip #4: Properly Manage Your Design Controls
The key to any successful design control process is clear documentation, organization, and communication. Make sure all team members are on the same page by creating concise documentation that’s understood by all. It’s also important to use a good software tool to manage your design controls. This will make it easier to track changes, ensure compliance with regulations, and generate accurate reports.
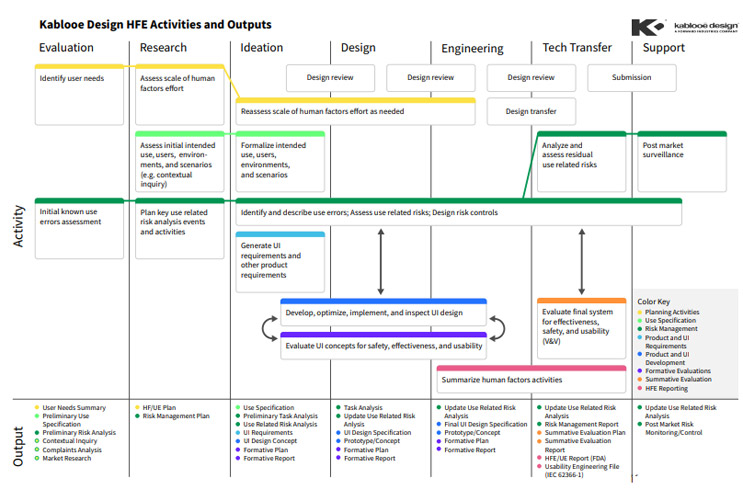
The Human Factors Engineering Plan
A human factors engineering (HFE) plan will help you think through how people will use your combination device in the real world and can help you avoid costly redesigns down the road. By integrating HFE activities into development at the start, you can mitigate a ton of issues that could pop up at the worst possible time.
A portion of this work will be covered in your design controls, which are governed by previously cited guidance documents, as well as the Association for the Advancement of Medical Instrumentation (AAMI) TIR59:2017.
Click on image to enlarge.
Pro Tip #5: Provide Your HFE Data BEFORE Your Summative Reports
The FDA wants to help you evaluate and form your summative protocol to make it more effective and with a higher chance of success — so let them!
Pro Tip #6: Avoid Human Factors Engineering Altogether
I know, this is contradictory to everything I just said. The thing is, though, if your usability risk assessment can show that HFE is not needed, you should make a good case for that and present it to the FDA. This can save you a lot of time and resources in the long run.
U.S. Vs. EU Markets
The FDA is not the EU, but it is close. The FDA and the European Medicines Agency (EMA) have different approval processes, so it’s important to understand the specific requirements of each one. Differences can include the types of studies required and the level of detail that needs to be included in your submission.
Here are some standards documents for reference:

Pro Tip #7: Understand the Regulatory Differences That Are Most Likely to Affect You
There are three main differences that are important enough to highlight here. Keep in mind, though, that these are general differences and not hard and fast rules. Things change fast.
The European system asks for a “User Interface Specification”. The U.S. guidance describes tasks to be done but stops short of requiring a specific report for those tasks that align.
The U.S. systems seem to need to see a lot of summative preliminary work. Europe, on the other hand, is more focused on the summative report.
The U.S. system puts a lot of focus on analysis such as Use Failure Modes and Effects Analysis (uFMEA) and fault tree analysis (FTA). The purpose of a uFMEA evaluation is to assess aspects of the user interface as well as task failure effects. An FTA examines assumptions to see whether they are reasonable or if modifications should be made.
Are You Ready To Make The FDA Happy?
Sure, you are! By following these seven pro tips, you’ll be well on your way to success. After all, making the FDA happy is essential for any medtech company looking to bring their combination device to market.
Want one final pro tip? Making the FDA happy isn't just about compliance — it's about elevating patient care and driving innovation in the medtech industry.
About The Author:
 Tom KraMer, president and CEO of Kablooe Design, has been a product innovator for over 30 years. He holds a certificate in master of product development from Northwestern University and a bachelor’s degree in industrial design from the Minneapolis College of Art and Design (MCAD), as well as a certificate from Stanford University’s Cardiovascular System in Health and Disease program. KraMer has created revenue for countless customers by delivering innovative product solutions to their portfolios. He spearheaded the D3 Process (Design Driven Development), a vehicle to provide these results to customers, and he teaches this process by traveling as a lecturer and speaking about innovation and development processes.
Tom KraMer, president and CEO of Kablooe Design, has been a product innovator for over 30 years. He holds a certificate in master of product development from Northwestern University and a bachelor’s degree in industrial design from the Minneapolis College of Art and Design (MCAD), as well as a certificate from Stanford University’s Cardiovascular System in Health and Disease program. KraMer has created revenue for countless customers by delivering innovative product solutions to their portfolios. He spearheaded the D3 Process (Design Driven Development), a vehicle to provide these results to customers, and he teaches this process by traveling as a lecturer and speaking about innovation and development processes.