Improving Drug-Device Combination Product Co-Development
By BioPhorum

In recent years, combination products have revolutionized the healthcare landscape, offering innovative and powerful treatment options that address a wide range of disease states. These products, which combine pharmaceuticals and medical devices, are not only transforming patient care but are driving rapid advancements in science and medicine.
Within the growing biopharmaceutical market, the need for drug delivery devices is predicted to grow substantially. Research suggests that the global drug delivery devices market (including inhalation, transdermal, injectable, etc.) was valued at $42.71 billion in 2023 and is projected to reach $96.34 billion by 2032.1 Even when we focus on an individual type of device, the projected growth of the market is significant – one study has predicted that the global market for autoinjectors will grow from $102.51 billion in 2022 to $233.69 billion by 2030.2
As the market for combination products matures, competitive pressures are increasing. This, along with the growing prevalence of biosimilars and the experience gained by organizations navigating the device-specific regulatory process, has led to a trend where novel medicines are often introduced as part of a combination product from the outset. This approach is becoming more common as companies seek to differentiate their products and offer more comprehensive treatment solutions.
The effectiveness of pharmaceuticals hinges on their safe and precise delivery to patients. Therefore, the reliability and efficiency of the associated devices are paramount. The integration of drug and device components allows for more targeted and controlled delivery of medications, which can significantly improve therapeutic outcomes.
Prefilled syringes and autoinjectors are currently the most commonly used combination products. These devices are widely recognized for their ability to reduce dosing errors and enhance patient adherence, as well as their convenience and ease for end users.
For instance, an autoinjector designed for self-administration of biologics or other medications ensures precise dosing and ease of use, thereby enhancing patient adherence and reducing the risk of dosing errors. However, the companies that produce them face a different reality. The life cycle of combination products involves intricate processes and high expectations. From initial design and specification through development, fabrication, assembly, and filling to processing, sterilizing, testing, labeling, and packaging – each stage presents unique challenges and complexities.
A Multidisciplinary Approach
The development of combination products is a complex process that requires precise coordination of components and interfaces to optimize performance and efficacy. This process necessitates a multidisciplinary approach involving experts in pharmaceutical sciences, engineering, regulatory affairs, and clinical research. The early recognition and mitigation of integration difficulties are critical to the success of drug-device combination products.
Moreover, the collaborative effort behind the development of these products cannot be overstated. The intricate interplay between drugs and devices presents significant challenges during product development. Effective collaboration among various experts ensures that both the drug and device components work seamlessly together to provide effective and safe treatment for patients. This integrated approach is essential for tackling the regulatory challenges that arise due to the multidisciplinary nature of combination products.
Timing And Coordination Challenges
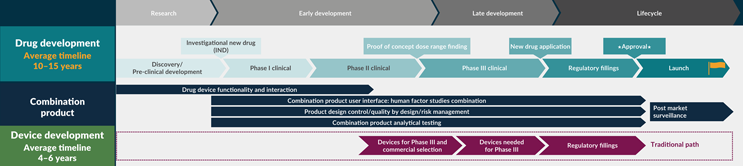
One of the significant challenges in developing combination products lies in the timing and coordination of device development. Historically, device development teams are often not consulted until after a therapeutic program has demonstrated success in a Phase 2B study (see Figure 1). This late involvement can put significant pressure on the device development schedule, making it a critical aspect of the overall project timeline.
Figure 1: The traditional timing for device development team involvement. Click on image to enlarge.
Organizations have alluded to a lack of device representation at the levels above that of senior director. This can often mean that significant decisions — such as those on coordination between drug and device development programs — are made without a full understanding of the complexity of device development.
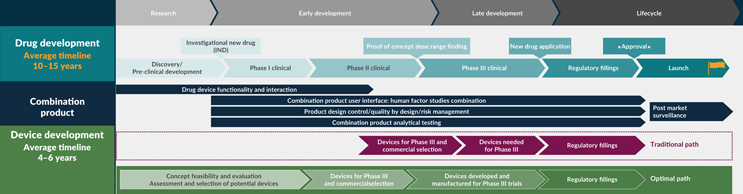
An Optimal Path In Device Development
Biopharma companies are increasingly recognizing the benefits of adopting a more optimal device development pathway. By integrating the device teams early in the drug development process, companies can ensure that the device team has full visibility of the drug pipeline. This approach allows the device development teams to function as stand-alone entities with executive leadership representation, rather than being subservient functions. Consulting the device team before a formulation exists enables them to influence key decisions and anticipate device-related challenges, embedding flexibility in the device design space and maintaining involvement throughout the clinical development process.
Also, when the formulation team is aware of the limitations of the device portfolio and platforms, it helps reduce risks and allows more time for device development. A full appreciation of the device development timelines is essential, especially considering the increasing complexity of devices. This holistic approach not only enhances the efficiency and effectiveness of the development process but also ensures that the final product meets the highest standards of safety and efficacy.
The device development process is governed by design controls, which are not only a legal requirement by the U.S. FDA but also serve as an effective system for project management. The development process is divided into four stages: research, early development, late development, and life cycle management. The journey begins with early concept and feasibility work, focusing on understanding user needs (see Figure 2). This is followed by project planning, where a design and development plan is created. This plan is dynamic and must be updated as new information is learned throughout the development process.
Figure 2: The device development process. Click on image to enlarge.
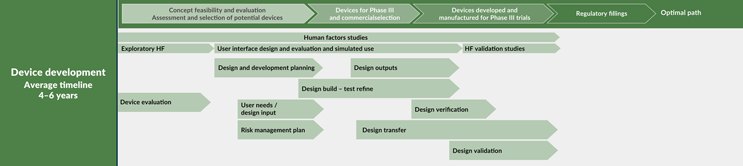
Clearly defining user needs is essential to the success of combination products in the marketplace. These needs are translated into design inputs, which are then refined through an iterative cycle of design, build, and test, all while considering human factors (see Figure 3). This meticulous process ensures that the final product meets the highest standards of safety, efficacy, and user satisfaction.
Figure 3: Device development life cycle. Click on image to enlarge.
Practical Actions For Improving Drug-Device Co-Development
One of the common challenges in the combination product industry is the lack of understanding of the requirements for developing a combination product. This includes areas such as development processes, human resources, cross-functional coordination, budgeting, and timelines. The earlier the device needs are considered in the drug development process, the more efficient the development of the combination product is and the more opportunity there is to address any drug-device integration and regulatory challenges.
These issues highlight the importance of early and continuous collaboration between drug and device development teams. By fostering a culture of cross-functional coordination and communication, companies can streamline the development process and ensure that both components are seamlessly integrated. This approach not only enhances the efficiency of the development process but also improves the overall quality and performance of the final product.
To overcome the challenges in drug-device co-development and improve timelines, it is essential to adopt a proactive and integrated approach. Here are some key strategies:
Ensure Early Involvement Of Device Teams
Device development teams should be involved early in the drug development process, ideally before a formulation exists. This allows for better alignment and anticipation of device-related challenges, reducing the risk of delays and ensuring that the device is not always on the critical path. Early involvement helps in understanding the drug's properties and requirements, allowing the device team to design and develop a compatible and effective delivery system.
Create Integrated Development Plans
Develop integrated project plans that consider both drug and device development timelines. This ensures that both components are developed in parallel, reducing the risk of misalignment and improving overall project efficiency. Integrated plans facilitate better coordination and communication between teams, ensuring that both drug and device development progress smoothly and are coordinated.
Foster Cross-Functional Collaboration
Foster a culture of collaboration between drug and device development teams. Regular communication and joint decision-making can help identify potential issues early and develop strategies to address them. Cross-functional collaboration encourages knowledge-sharing and problem-solving, leading to more innovative and effective solutions.
Ensure Regulatory Alignment
The regulatory landscape for combination products is complex, with increasing skepticism toward bridging studies and a preference for using the commercialized device in clinical studies. Ensure that regulatory and clinical development teams are aligned from the outset. This helps when navigating complex regulatory requirements and avoiding delays due to miscommunication or misalignment. Regulatory alignment ensures that both drug and device components meet the necessary standards and requirements, facilitating a smoother approval process.
Begin Exploring Human Factors Considerations Earlier
Human factors often become an afterthought, leading to significant challenges in the final stages of product development. Human factors refer to the study of how users interact with devices, focusing on usability, safety, and effectiveness. When these considerations are not integrated early in the development process, it can result in devices that are difficult to use, prone to errors, and potentially unsafe. This oversight can lead to costly redesigns, delays in regulatory approval, and, ultimately, a product that fails to meet user needs and expectations. By prioritizing human factors from the outset, companies can ensure that their combination products are not only technically sound but also user-friendly and safe, leading to better patient outcomes and more successful market entry.
It is essential to start exploratory research early in the development process and conduct validation studies as close to regulatory filing as possible. This approach helps anticipate and address potential usability issues and align device development with drug properties such as viscosity.
The importance of this timing cannot be overstated. By incorporating human factors engineering principles early in the development process, companies can design devices that are intuitive and easy to use, reducing the likelihood of user errors and improving overall patient outcomes.
Enhance Leadership And Visibility
Ensure that senior individuals with device development expertise are involved in strategic decision-making processes. When senior individuals are part of the leadership team, it enhances their visibility within the organization, which can help when advocating for the importance of device development and ensuring that it receives the attention and resources it deserves. This enhances leadership and visibility, helping to integrate the development of combination products and lower overall program risk. Involving senior individuals with device development expertise in strategic decision-making processes ensures that their insights and knowledge are integrated into the company's overall strategy. This can lead to more informed and effective decisions that align with the company's goals and objectives.
Embed Flexibility In Device Design
Embed flexibility in the device design space and stay involved throughout the clinical development process. This allows for adjustments and improvements based on clinical feedback, evolving drug properties, and real-world use. Flexibility in design ensures that the device can adapt to changes and meet the requirements of different drug formulations and patient needs.
Ensure Awareness Of Device Limitations
Ensure that the formulation team is aware of the limitations of the device portfolio and platforms. This helps when making informed decisions about drug formulation and delivery methods, reducing the risk of compatibility issues. Awareness of device limitations allows for better planning and coordination, ensuring that the chosen device can effectively deliver the drug.
Address Risk Management Proactively
Reduce risk by allowing more time for device development and appreciating the complexity of device development timelines. Proactive risk management involves identifying potential challenges early and developing strategies to mitigate them, ensuring a smoother development process.
By adopting these strategies, biopharmaceutical companies can improve the efficiency and effectiveness of their drug-device co-development programs, ultimately delivering innovative and powerful treatment options to patients more quickly and effectively.
Conclusion
There are strong signs of improvement within the industry as we see more license-holding organizations developing new structures to ensure that the complexity of device development is considered as early as possible when developing new drug products, during discovery and their early development. We are also seeing more organizations include a senior-level representation from the devices team, which ensures that device development teams receive the attention and resources they require. Nevertheless, there is still room for optimization and better integration of device development and device providers into drug programs.
Combination products have emerged as a cornerstone of modern healthcare, offering innovative solutions that improve patient outcomes and set new standards in treatment efficacy. Their development requires a collaborative, multidisciplinary effort and, as the market continues to evolve, the importance of these products is only expected to grow. By addressing the challenges in device development and adhering to rigorous design controls, the healthcare industry can continue to advance, offering even more powerful and effective treatment options in the future.
References
- Drug Delivery Systems Market Size, Share & Industry Analysis, Fortune Business Insights, March 2025, www.fortunebusinessinsights.com/amp/drug-delivery-systems-market-103070
- Autoinjectors Market Size, Share, Trends & COVID-19 Impact Analysis, Fortune Business Insights, Oct 2023, www.fortunebusinessinsights.com/autoinjectors-market-108507