India Looking To Local Solutions For Domestic Medtech Needs
By Gunjan Bagla, Amritt Inc.
 For most of the last century, innovation in the medical device ecosystem has emerged from rich countries: the United States, Japan, Europe, and Australia. But today, a thriving ecosystem has developed across India, driven in part by unique needs in India and in part by the cross-fertilization of ideas, education, and capital from American sources. In this article, we look at three startups that are addressing India’s unique problems today, but may eventually bring their solutions to a global audience.
For most of the last century, innovation in the medical device ecosystem has emerged from rich countries: the United States, Japan, Europe, and Australia. But today, a thriving ecosystem has developed across India, driven in part by unique needs in India and in part by the cross-fertilization of ideas, education, and capital from American sources. In this article, we look at three startups that are addressing India’s unique problems today, but may eventually bring their solutions to a global audience.
For VAP Sufferers, A Breath of Fresh Air
Nitesh Jangir spent nine months at the Stanford India Biodesign Program held in New Delhi. What he learned through the program formed the base on which he co-founded COEO Labs. He and co-founder Nachiket Deval, a graduate of India’s National Institute of Design, underwent a program at the National Institution for Transforming India that focused on innovation and entrepreneurship. They spent two months undergoing clinical immersion at a tertiary care hospital, along with several weeks in the ambulance and peripheral care settings. Unmet needs were identified and validated across parameters such as frequency, criticality, market size, regulatory pathway, and technical complexity. Finally, they selected ventilator associated pneumonia (VAP), a lung infection that happens to in-patients who are on mechanical ventilation breathing machines, as the focus for their efforts.
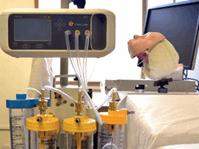 Funded by InnAccel, and the United States Agency for International Development,
Funded by InnAccel, and the United States Agency for International Development,  the COEO team designed an electronic machine called VAPCare — an intelligent, automated, closed-loop, secretion management system. The device can be powered through batteries, compressed gases, an ambulance’s DC electrical supply, or manually. In an email exchange, Deval said that the designers’ initial target market is Intensive Care Units at hospitals. COEO Labs just obtained regulatory approval for the device in India, and 10 devices have been sold as a pilot sale, Deval added.
the COEO team designed an electronic machine called VAPCare — an intelligent, automated, closed-loop, secretion management system. The device can be powered through batteries, compressed gases, an ambulance’s DC electrical supply, or manually. In an email exchange, Deval said that the designers’ initial target market is Intensive Care Units at hospitals. COEO Labs just obtained regulatory approval for the device in India, and 10 devices have been sold as a pilot sale, Deval added.
Addressing A Shocking Lack of Defibrillators
 According to the Global Burden of Cardiovascular Disease, cardiovascular diseases (CVD) have become a leading cause of mortality in India. In comparison with the people of European ancestry, CVD often affects Indians in their most productive midlife years while it generally affects people of European ancestry later in life." But over one billion of India’s 1.3 billion people do not have easy access to a defibrillator.
According to the Global Burden of Cardiovascular Disease, cardiovascular diseases (CVD) have become a leading cause of mortality in India. In comparison with the people of European ancestry, CVD often affects Indians in their most productive midlife years while it generally affects people of European ancestry later in life." But over one billion of India’s 1.3 billion people do not have easy access to a defibrillator.
The co-founders of Jeevtronics, (meaning ‘life electronics’), Ashish Gawade and Aniruddha Atre, both hail from India’s western state of Maharashtra. Gawade acquired an MS in Mechanical Engineering from Wayne State University, and an MBA in strategy and marketing from the University of Michigan. Atre holds a Bachelor and Master’s Degree in Mechanical Engineering, as well as an MBA from the University of Michigan. Their company makes a dual-powered defibrillator that can work on electricity, but also can be hand-cranked when or where there is no electricity (a situation common in India). This life-saving device only requires the administrator to power it up for less than 12 seconds by rotating a small hand paddle.
Wear Your Heart On Your Chest
Artificial Intelligence is increasingly being incorporated into connected medical devices and Indian startups have also taken this plunge. Take the case of ten3T that has built a wearable real-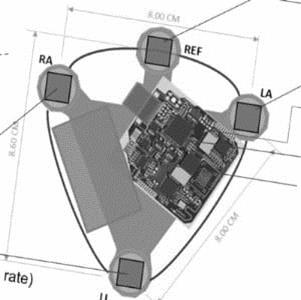 time monitoring chest patch that is wirelessly charged.
time monitoring chest patch that is wirelessly charged.  The 3 ½ inch triangular patch, Cicer, is a comprehensive patient monitoring system that measures a 6 lead electrocardiogram, heart rate, respiration, blood oxygen saturation and temperature. Data is transmitted in real time to the treating physician and monitoring team. Algorithms look for immediate signs as well as longer term subtle changes, allowing clinicians to make both immediate and predictive decisions. In a response to a query by email, co-founder and CEO of the Bangalore-based start-up, Dr. Sudhir Borgonha said, “We are ‘live’ in a number of hospitals and are seeing significant traction from patients and doctors. Our target is to facilitate continuous monitoring outside the ICU in mid-size, multi-specialty hospitals. We do not sell the devices but rent them out on a ‘per use’ or ‘per month’ model. As scale manufacturing is best done by a specialized vendor, we will also be doing so.” After obtaining his medical qualifications at St. John’s Medical College, Bangalore, Dr. Borgonha studied at the Sloan School of Management at the Massachusetts Institute of Technology. Along with Rahul Shingrani and Prasad Bhat, both biomedical engineers, ten3T was founded and is now being used in multiple clinical settings. The company raised funding from pi Ventures and angel investors from the Bay Area and Bangalore.
The 3 ½ inch triangular patch, Cicer, is a comprehensive patient monitoring system that measures a 6 lead electrocardiogram, heart rate, respiration, blood oxygen saturation and temperature. Data is transmitted in real time to the treating physician and monitoring team. Algorithms look for immediate signs as well as longer term subtle changes, allowing clinicians to make both immediate and predictive decisions. In a response to a query by email, co-founder and CEO of the Bangalore-based start-up, Dr. Sudhir Borgonha said, “We are ‘live’ in a number of hospitals and are seeing significant traction from patients and doctors. Our target is to facilitate continuous monitoring outside the ICU in mid-size, multi-specialty hospitals. We do not sell the devices but rent them out on a ‘per use’ or ‘per month’ model. As scale manufacturing is best done by a specialized vendor, we will also be doing so.” After obtaining his medical qualifications at St. John’s Medical College, Bangalore, Dr. Borgonha studied at the Sloan School of Management at the Massachusetts Institute of Technology. Along with Rahul Shingrani and Prasad Bhat, both biomedical engineers, ten3T was founded and is now being used in multiple clinical settings. The company raised funding from pi Ventures and angel investors from the Bay Area and Bangalore.
About The Author
Based in Los Angeles, Gunjan Bagla is managing director of Amritt Inc., a California-based consulting firm focused on helping American companies to succeed in India. His clients include Covidien, Roche Diagnostics, BD, Nordic Naturals, Johnson & Johnson, Gojo, and many more. Gunjan spoke three times at the MD&M West Conference in Anaheim, and was on the keynote panels at MEDevice San Diego and IMDI in Ahmedabad, India.
For his India expertise, he has appeared in The New York Times, the Los Angeles Times, and the Washington Post, and on Bloomberg TV, BBC Television, and Fox Business News. He also writes about India for the Harvard Business Review and the Huffington Post. Gunjan has an MBA from Southern Illinois University and a mechanical engineering degree from the Indian Institute of Technology (IIT) Kanpur in India.
