4 Key Strategies When Designing Surgical Robotics
By Dvir Cohen, cofounder & CEO, Memic Innovative Surgery Ltd.

Since the FDA approved the first robot-assisted surgical platform for general laparoscopic procedures more than 20 years ago, surgical robotics has completely transformed the field of minimally invasive surgery. Robotic assistance helps surgeons make more precise movements and allows surgeries to be done less invasively. Patients have realized significant clinical benefits as a result, including less pain and shorter recovery times. These and other advantages have led to the significant growth of the surgical robotics industry in recent years, with increased clinical adoption across global markets and more manufacturers entering the field with the goal of introducing novel technologies that may further improve surgical techniques.
However, surgical robotics is a relatively new concept, and many clinicians are still discovering the optimal surgical techniques for different procedures and, simultaneously, manufacturers are learning what clinicians really need – and what hospitals and medical facilities can afford. Based on my years of experience in developing innovative robotic systems, I have learned that the important “make or break” decisions of any medical device manufacturer are often made at earlier stages, such as the concept or design phase. These are the crucial stages that can determine FDA approval, market adoption, and overall success. Following are four key strategies that device manufacturers must consider when designing or developing surgical robotics.
1. Analyze The Market
The purpose of robotics is to bring new tools and instruments that change the game and provide surgeons with more capabilities, with the goal of ultimately improving patient care. Given the rapid growth of the surgical robotics industry, it is important to first analyze the target market to understand what robotic technologies are currently available. As more and more competitive devices appear on the market and are in development toward commercial release, it is essential that device manufacturers differentiate their product from existing competitors and future products to enhance utility and support customer adoption post-launch. Efforts might also involve market research studies that assess the size of the target patient population, the likelihood of clinician adoption and payer reimbursement, and the potential strengths of a product’s clinical and economic value propositions.
Manufacturers must also understand clinical unmet needs and how surgical robotics might be able to fulfill those needs, which often involves having proactive discussions with strategic players in the field, including universities, academic centers, medical organizations, and leading clinicians. These different groups can provide valuable insights about the real-world trends or issues they are seeing with surgical robotics to help identify opportunities for new technologies to optimize the value of bringing something entirely different to the market. For example, in early development of our Hominis Surgical System, we realized from early discussions that there was an unmet need in gynecologic surgery. Surgeons reported that they prefer to use the vaginal approach in gynecological procedures such as hysterectomies because going through a natural orifice is shown to provide optimal clinical outcomes for patients, minimizing trauma to the abdominal wall and promoting faster recovery and healing. At the time, I also saw a statement from the American College of Obstetricians and Gynecologists (ACOG) that stated, “vaginal approach to hysterectomy is the approach of choice whenever feasible.” However, surgeons only use this approach in about 16% of cases1 for two primary reasons. First, a vaginal hysterectomy is technically more challenging due to the lack of visualization. Second, there are several anatomic aspects of the disease state that prohibit use of the vaginal approach, even if a surgeon has the necessary training and skills. We realized that there was a significant opportunity to revolutionize the field and provide innovative robotic technology that would enable more surgeons to provide more women the preferred surgical approach for their hysterectomy. This unmet need guided our future decisions in developing Hominis.
2. Find The Right Partners
It is essential that device manufacturers, including those focused on surgical robotics, surround themselves with the right partners who support their vision and provide the talent and expertise needed to take a product from concept through development and potential commercialization. Valuable partners might range from universities, academic institutions, and leading clinicians in the target field of interest, as mentioned previously, to contract research organizations, third-party vendors, and even regulators.
When our team at Memic completed design and manufacture of the Hominis System, we had to begin thinking about developing supporting data through clinical trials. We did our due diligence to determine which surgeons in the field might be the best partners to support these efforts. Our research led us to collaborate with Dr. Jan Baekelandt, a gynecologic oncologist at Imelda Hospital in Belgium, and Dr. Lior Lowenstein, a gynecology and obstetrics surgical specialist and deputy chairman of the Department of Gynecology at Rambam Hospital in Israel. Based on their extensive academic work and peer-reviewed studies in gynecologic surgery, we knew they would understand our goal with Hominis and provide the optimal support and insights needed to successfully conduct clinical trials. For example, Dr. Baekelandt is a pioneer in the emerging field of natural orifice surgery and completed his Ph.D. on transvaginal natural orifice transluminal endoscopic surgery (vNOTES), of which he has performed more than 1,500 procedures. He introduced vNOTES in Europe, developed the technique of total vaginal NOTES hysterectomy and of vaginal retroperitoneal sentinel node dissection, and introduced transvaginal robotic surgery as part of his Ph.D. research. His experience was integral to our journey toward enabling surgeons to perform the vaginal approach in gynecologic surgery using Hominis.
Technology and device manufacturers must also position themselves to maintain open and frequent communications with target customers and end users, encouraging long-lasting partnerships. Their continued guidance can ensure that manufacturers stay up to date on the latest developments in the field and remain educated on areas for improvement related to their product. Partner engagement can also help find advocates of innovative surgical robotics to increase the likelihood of adoption. It is best to start discussions with potential partners as early as possible in the development process.
3. Focus On Simplicity, Not Complexity, For The End User
Most currently available commercial robotic systems are controlled with large, complex, and expensive equipment that dominates the available space in operating rooms and limits accessibility to the patient in surgery. As a result, many hospitals and ambulatory surgery centers, the latter of which often have limited budgets and space, are unable to purchase large, costly, and cumbersome equipment.
It is important in developing any device or surgical robot to keep the end user top of mind throughout the process. There is always a learning curve for surgeons when using a new device, but to enable rapid and widespread adoption, devices must be simple, reproducible, and easily teachable for other surgeons to be willing to adopt the technology.
In assessing the surgical robotics market, we found that current robotic instruments are limited to straight shaft and single wrist designs. Straight shaft instruments must be introduced through multiple incisions in the body – which is similar to laparoscopic surgery without robotics. I thought there must be a different and easier approach and asked myself what if there was a way to develop a robot that performs as if the surgeon is miniaturized and placed inside the body? That is how the idea originated of a robotic system that has miniature humanoid-shaped robotic arms that mimic the entire upper extremity, with shoulder, elbow and wrist joints. In discussions with different surgeons in gynecology, urology, general surgery, and other therapeutic areas, I found consensus in the idea of a miniature humanoid-like robot that could mimic the flexibility, dexterity, and 360-degree articulation of a surgeon with fingers, rather than using forceps and graspers that present limitations in mobility.
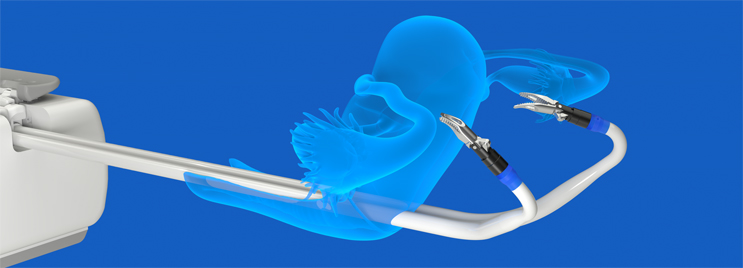
We also designed Hominis so that the surgical workflow is in the same anatomic direction (fundus to cervix or top to bottom of the uterus) that surgeons are familiar with in laparoscopic, robotic, or even open gynecological procedures. Figuring out how to add value to standard or even advanced practices can distinguish you from the competition and designing a product that is simple and easy to use for the customer sets the bar for success.
The cost of adoption is another important factor to consider, which is why our team also focused on making Hominis small and compact, and thus a fraction of the cost compared to other robotic technologies. The capital equipment required is so small that the entire system can fit in the palm of your hand, and it is attached to the surgical table, which improves usability and access to the patient. The small, compact design also provides simple docking and a short setup time for surgeons and its minimal footprint allows it to be used it in any size operating room, including ambulatory surgery centers.
4. Build For Future Indications
Identifying an unmet clinical need is key in developing any surgical robot but it is also important to think about the long-term future applications. Manufacturers must consider designing a product that has the potential to expand and add value in new indications so the technology becomes a platform technology and can be used for multiple procedures and even by multiple surgical specialties. In developing Hominis, we understood early on that its unique differentiating feature was the humanoid-shaped robotic arms, and that the unprecedented level of flexibility and dexterity they provide could potentially be applied to other surgical areas. As a result, we plan to pursue additional indications, including general surgery indications through abdominal and transluminal approaches and indications that have historically been off-limits to robot-assisted surgery.
Conclusion
An innovative idea is just the starting point. Manufacturers must develop a strategic and long-term oriented business plan early on to ensure success down the road. Efforts will involve close and frequent collaboration with reliable and experienced partners, a dedicated team of people who share your vision and can contribute to it, a comprehensive understanding of the unmet clinical need of end users, and clear business processes to reach the unreachable.
References:
- Luciano AA, Luciano DE, Gabbert J, Seshadri-Kreaden U. The impact of robotics on the mode of benign hysterectomy and clinical outcomes. Int J Med Robot. 2016 Mar;12(1):114-24. doi: 10.1002/rcs.1648. Epub 2015 Mar 4. PMID: 25753111.
About The Author:
 Dvir Cohen is cofounder and CEO of Memic Innovative Surgery Ltd., a medical device company founded in 2013 in Tel Aviv, Israel, that has a wholly owned subsidiary in Fort Lauderdale, FL. Cohen has more than 15 years of experience in the development and manufacture of robotic systems. From 2011 to 2013, he was an R&D manager and robotic project specialist in an elite technology unit of the Israel Intelligence Force. From 2007 to 2011, he led several optomechanical disruptive solutions in the Ministry of Defense, one of which was awarded the Israel Defense Award. Cohen’s recent recognitions include receiving the Deal of the Year Award from BioFlorida and being named an Endeavor Entrepreneur by Endeavor Miami. He earned an MBA from the Recanati Business School at Tel Aviv University. He also earned a B.S. and M.S. degree in mechanical engineering from the Technion.
Dvir Cohen is cofounder and CEO of Memic Innovative Surgery Ltd., a medical device company founded in 2013 in Tel Aviv, Israel, that has a wholly owned subsidiary in Fort Lauderdale, FL. Cohen has more than 15 years of experience in the development and manufacture of robotic systems. From 2011 to 2013, he was an R&D manager and robotic project specialist in an elite technology unit of the Israel Intelligence Force. From 2007 to 2011, he led several optomechanical disruptive solutions in the Ministry of Defense, one of which was awarded the Israel Defense Award. Cohen’s recent recognitions include receiving the Deal of the Year Award from BioFlorida and being named an Endeavor Entrepreneur by Endeavor Miami. He earned an MBA from the Recanati Business School at Tel Aviv University. He also earned a B.S. and M.S. degree in mechanical engineering from the Technion.
