Kits, Packaging Configurations, And Unit Of Use – A UDI Primer
By Dan O'Leary, President, Ombu Enterprises

I often receive questions to clarify the difference among kits, packaging configurations, and unit of use. (See the Comments section of my previous article A Guide To Device, Label, And Package Requirements Of The UDI Rule for examples.) These questions usually inquire about labels or entering data into GUDID. This article answers these questions in simple terms, without citing the regulations. The article provides the “mental models” you need to distinguish among the cases and develop a compliant approach.
The Three Cases
The first step determines the type of product you have and which model to apply. While the UDI rule has formal definitions, they don’t add clarity until you grasp the concepts.
A kit is a collection of devices sold together to make it easier for the customer. The kit contains two or more different devices in the same package. For our purposes, each device in the kit has a label with the device identifier (DI) for the device. In addition, the kit has its own DI.
Example #1a: A doctor diagnoses a patient with Type II diabetes; the doctor asks the patient to check her blood glucose every day. The doctor prescribes a starter kit with a four different devices: a blood glucose meter, a lancing device to hold the lancet, a package of lancets, and a package of blood glucose test strips.
Example #1b: This is a kit with multiple devices inside. The important point is that the kit’s label will have its own DI. In addition, each of the devices inside the kit will also have a DI. (Note: Some of the devices will also have a unit of use DI, but that is not relevant at this point.)
In contrast, a packaging configuration contains two or more of the same device in the same package. There is a fixed quantity of devices (e.g., a five-pack). Each device in the packaging configuration has a label with the DI of the individual device. In addition, the packaging configuration has its own DI.
Example #2: The patient with Type II diabetes, described in Example #1, goes to the local pharmacy to fill the prescription for the starter kit. She gets one kit, which has a DI on the kit’s label. The pharmacy, however, doesn’t order the kits one at a time. Instead, they place an order for a case of 20 kits. This is a packaging configuration, and the labeler (the kit manufacturer) assigns a DI to the case of 20 kits.
In the unit of use case, the individual devices are all the same, dispensed from the package, and don’t have a label with the DI. The dispensing package has a DI on the label, but the individual devices are not marked with a DI. Nonetheless, each individual device has a DI, even though it is not marked. An electronic health record (EHR) needs to distinguish between using just one lancet for a patient and using the whole box on the patient.
Example #3a: The patient opens up the kit and sees the four items: a blood glucose meter, a lancing device, a package of lancets, and a package of blood glucose test strips. (She will take the package to the nurse to learn how to use the kit. The nurse enters the kit’s UDI into the patient’s EHR.)
Example #3b: Because this is a starter kit, the box of lancets holds 25 units. The package has a DI, but the individual lancets do not. Each individual lancet is a unit of use, because the patient dispenses them daily from the box.
Example #3c: Because this is a starter kit, the vial of test strips holds 25 units. The vial has a DI, but the individual test strips do not. Each individual test strip is a unit of use, because the patient dispenses them daily from the vial.
The Differences
In simple terms, the differences are:
- The kit contains different devices in the package.
- The packaging configuration contains a fixed quantity of the same devices in the package.
- The unit of use contains a fixed quantity of the same device in the package and they remain inside until dispensed, usually one at a time.
The Pharmacy’s Purchases
The pharmacy will stock blood glucose starter kits, lancets, and glucose test strips. Once the patient uses up the consumables in the starter kit, she will buy lancets 100 to a box and test strips 50 to a vial.
The pharmacy purchases the lancets in cases of 125 boxes and the test strips in cases of 75 vials.
In summary, the pharmacy’s purchasing department places orders for cases, not kits, boxes, or vials. If the pharmacy wants 250 boxes of lancets for inventory, they would issue a PO with the part number for one case and a quantity of two. If they used the part number for one case and quantity of 250, they would get 250 cases or 250 × 125 = 31,250 boxes.
The cases are, from the manufacturer’s point of view, a packaging configuration with a fixed quantity of the same device in the case. The packaging configuration will have a DI at the case level, which will correspond with the manufacturer’s catalog number for one case.
The Hierarchy of DIs
The DIs for all of these devices form a structure. The illustrations use simple, three-digit DIs. In practice, they are much longer and could have both numbers and letters.
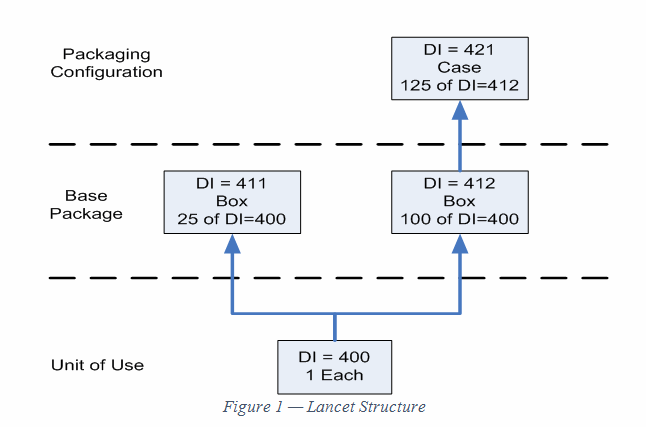
Figure 1 shows the structure for the lancet. At the unit of use level there is a DI assigned to each lancet. There are two different DIs at the Base Package level, because there are two different quantities. At the Packaging Configuration level, the DI represents 125 boxes of one of the base packages.
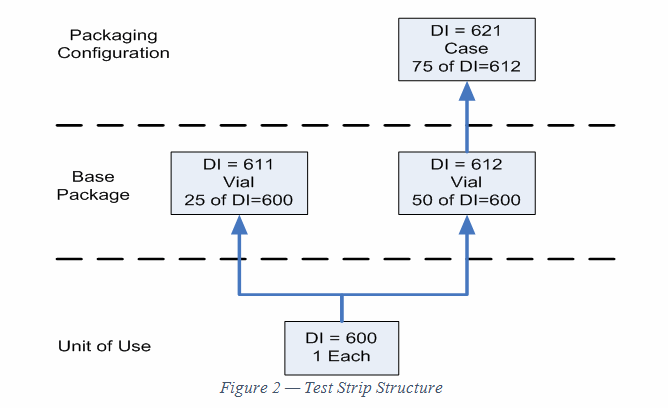
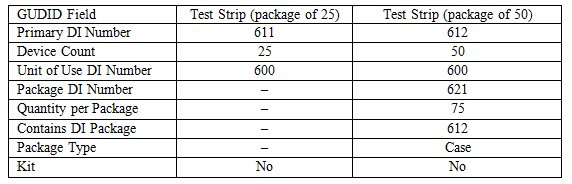
Figure 2 shows the structure for the test strip. It is similar to the lancet structure, but the DI and quantities are different.
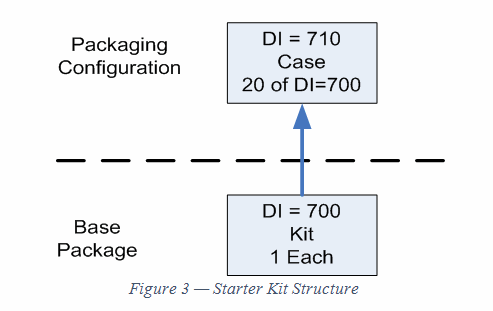
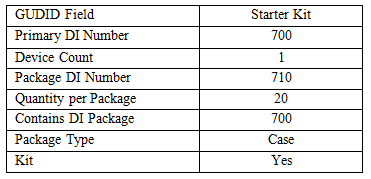
Figure 3 is the structure of the starter kit. It does not have a unit of use; the lowest level is the kit. Also, notice that, in terms of the UDI rule, the contents of the kit don’t appear. Only the kit and its packaging configuration matter for UDI.
Typically, the devices in the kit would each have a DI on their label, but under some circumstances, they are exempt.
Loading GUDID
In terms of devices, there are five: a blood glucose meter, a lancing device to hold the lancet, a lancet, a blood glucose test strip, and a starter kit. However, there will be seven DI records in GUDID and 12 DIs in total.
Start with the blood glucose meter and the lancing device. We did not analyze them for packaging configurations and they don’t have a unit of use, so this is a simple approach.
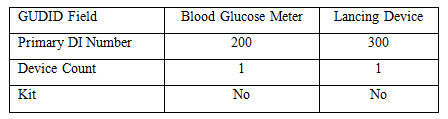
For the lancet, we need two DI records. There are two base packages since each has a different quantity. When the DI record doesn’t have an entry, the field has a dash (–).
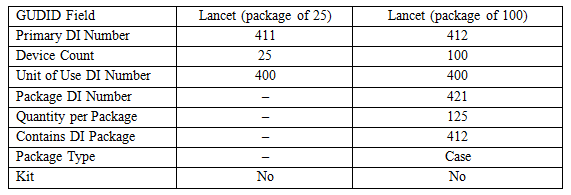
The DI record tells us a few important things. Because the base packages have different quantities, there must be two DI records. Both base packages use the same individual lancet. Since the manufacturer doesn’t sell the lancets one at a time, it doesn’t have its own DI record; it is not a primary DI.
Notice also the structure of the packaging configuration. It has a DI (but not a primary DI) and the record tells how many of the next lower DI is in the packaging configuration.
For the test strip, the structure is similar.
The kit has a packaging configuration, but not a unit of use; the device count is 1.
Summary
The three concepts of kit, packaging configuration, and unit of use share some common characteristics. They all represent collections of devices. However, the devices in the collection expose the differences. The analysis always starts with the base package, and then extends up or down, as necessary to capture the differences.
Other UDI-related articles by this author include:
