LidoSite Now Available To Patients In Physician Offices
Fair Lawn, NJ - Marking an advance in the widespread distribution of rapid pain relief from procedures involving venipuncture, intravenous cannulation and laser ablation of superficial skin lesions, Vyteris, Inc. announced the commercial availability of LidoSite to the 40 million American patients in physician offices who are needle averse or even phobic. 
"This marks a significant step in the treatment of pain resulting from needles and laser ablation and provides physicians and patients a new and powerful tool in improving patient comfort," said Timothy J. McIntyre, president and chief executive officer for Vyteris, Inc. "As a fast-acting alternative to topical creams or simply trying to ignore needle pain, we believe LidoSite will be well-received by patients and physicians."
With close to one billion blood draws taken a year in the U.S., according to a study by market research firm TVG, it is estimated that each year approximately 40 million American patients experienced "high discomfort" with blood draws due to needle pain1. An overwhelming majority of the surveyed patients who experienced "high discomfort" – 65 percent indicated they intended to use or ask for LidoSite at their next blood draw – representing an initial potential U.S. market for LidoSite of 26 million patients annually for this segment alone.
Working alongside its marketing partner in Laboratory Corporation of America Holdings (LabCorp) in the general practice physician market, Vyteris also announced the retention of experienced specialty sales representatives to make LidoSite broadly available in high demand specialties such as rheumatology, oncology and dermatology.
"Launching LidoSite into the U.S. physician office market represents a strategic and significant accomplishment for Vyteris by offering the first real, scalable product commercialization by the company on its own," McIntyre said. "This knocks down yet another important strategic benchmark outlined to our shareholders at the beginning of the year and is another important step in our three-year growth plan."
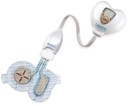
LidoSite is the first active transdermal patch approved by the U.S. Food and Drug Administration to deal with pain associated with blood draws (venipuncture) IV (intravenous) cannulations and laser ablation of superficial skin lesions. The device uses the process of iontophoresis to carry a combination of the local anesthetic lidocaine and vasoconstrictor epinephrine, which localizes the anesthetic effect, from a patch through the skin layer and into the underlying tissue by way of a mild electrical current.
LidoSite is composed of a heart-shaped controller that houses a microprocessor regulating dosing of medication, intervals and duration of dosing and is good for up to 99 patch applications.
The use of LidoSite is supported by the Blue Cross/Blue Shield Technical Evaluation Center (TEC), which indicated that "use of iontophoresis to administer local anesthetic before skin puncture or dermal procedures meets the TEC criteria." Various Blue Cross/Blue Shield health insurance plans have also previously issued medical practice guidelines supporting TEC findings and indicating that "iontophoresis is considered medically necessary to administer local anesthesia prior to a venipuncture or dermatological procedure."2
The American Pain Society (APS) and the American Academy of Pediatrics (AAP) set forth guidelines3 for adequately addressing acute – or short-lived – pain. These guidelines, which apply to the treatment of children and adults, call for healthcare practitioners to eliminate or reduce pain caused by medical treatments whenever possible. In fact, the APS and AAP agree that acute pain experienced with medical procedures can, in most cases, be substantially reduced and even prevented.
Please refer to the full Prescribing Information, including the detailed instructions for use, before using the LidoSite topical system.
1 TVG, Inc., November 2006, 1,010 respondents, +/- 3.5 percent
2 Blue Cross Blue Shield Association Technology Evaluation Center (TEC) Iontopheresis for Medical Indications. Assessment Program, Volume 18, No. 3. June 2003. Available at http://www.bcbs.com/tec/vol18/18_03.html
3 Position Statement from the American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health and American Pain Society Task Force on Pain in Infants, Children, and Adolescents, 2001, Online: http://www.ampainsoc.org/advocacy/pediatric2.htm.
SOURCE: Vyteris, Inc.
