Long-Lasting Injectable CGM Uses Live Cells To Regenerate Sensor
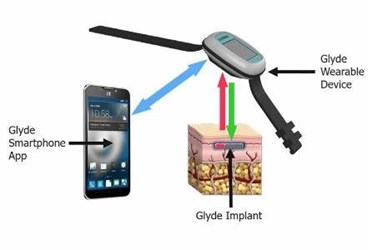
The Juvenile Diabetes Research Fund (JDRF) has invested in a long-lasting continuous glucose monitor (CGM) that uses engineered live cells to replenish and maintain the stability of the implant’s optical biosensor over time. The GluSense Glyde, a product of Rainbow Medical, is projected to last up to one year under the patient’s skin, disrupting the current market of CGMs that last between seven and 90 days.
The JDRF, a global leader in research funding for type 1 (TD1) and type 2 (TD2) diabetes, recently launched the JDRF TD1 Fund with $42 million in pledged commitments from the foundation and outside donors to fund the development of breakthrough commercial TD1 therapies. Jonathan Behr, managing director of the initiative, projects raising an additional $80 million over the next two years.
“Research advances in TD1 are accelerating at an unprecedented pace,” said Behr in a press release. “T1D research is at a critical inflection point, and the T1D Fund has been created to catalyze major investment in the commercialization of the great research discoveries of the last decade.”
One of those investments has been allocated for the minimally invasive GluSense Glyde, which is injected under the skin using local anesthesia and transmits glucose levels wirelessly to a wearable device, according to a press release. The system uses a proprietary fluorescent biosensor, “[ensuring] accuracy at the medically-important hypo glucose range” and maintaining stability over the long term by integrating engineered live cells that continuously replenish the implant’s optical biosensor. The “ground-breaking technology” ensures fewer replacements and fewer finger-prick calibrations, said developers.
“Today, diabetic patients need to endure frequent finger pricks daily in order to manage their glucose level, and even with modern CGMs, frequent calibrations, measurement verifications and replacements are still a hassle and limit efficient treatment,” said GluSense CEO Boaz Brill in a press release. “In contrast, our Glyde CGM will provide accurate glucose measurement for a full year with significantly fewer blood glucose calibrations.”
GluSense is an Israel-based startup that is part of the Rainbow Medical innovation house, which funds and develops medtech startups across a range of medical fields, addressing unmet needs. Last year, Rainbow Medical launched a joint venture with Johnson & Johnson to develop a novel therapy for degenerative disc disease. Rainbow also inked a deal with Medtronic and Abbott on an injectable neuro-stimulation device, BlueWind.
JDRF’s investment in GluSense, comprising an undisclosed amount, will bring the Glyde closer to human clinical trials, which will be used to apply for regulatory approval for commercial launch.
“The GluSense Glyde CGM … when paired with future advanced artificial pancreas systems or insulin injections, it will reduce the burden of T1D and help those living with the disease maintain blood glucose levels in safe range,” said Behr.
An implantable CGM sensor developed by Senseonics, which lasts at least 90 days once implanted, was recently submitted to the FDA and is already approved for use in Europe.
