Medtronic Touts Success Of Tiny, Leadless Pacemaker
By Jof Enriquez,
Follow me on Twitter @jofenriq
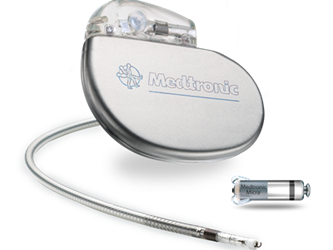
During the 2015 American Heart Association Scientific Sessions, Medtronic announced promising global clinical trial results of the company's tiny, leadless pacemaker – the Micra Transcatheter Pacing System (TPS) – which demonstrated high successful implant rates and low complication rates compared to conventional pacemaker devices.
According to study findings presented at the meeting and published in the New England Journal of Medicine (NEJM), the Micra device was successfully implanted in 719 of 725 patients, or 99.2 percent of participants, and registered a Kaplan–Meier estimate (rate of the primary safety endpoint) of 96 percent. There were 28 major complications in 25 patients, although those patients had significantly fewer major complications compared to 2,500 historical control patients with conventional pacemakers.
“We are extremely pleased with the remarkably successful implant rates and safety profile of the Micra pacemaker, including the absence of device dislodgments. We are especially confident in these results because the trial included patients with serious comorbidities from 19 countries on five continents around the world,” writes Dwight Reynolds, M.D., trial principal investigator and regent’s professor and chief of the Cardiovascular Section at the University of Oklahoma Health Sciences Center, in a statement.
According to Medtronic, 292 of 297, or 98.3 percent of patients, achieved low and stable pacing thresholds at six months, yielding projected average longevity for the device of more than 12 years (300 patients at six months). Compared to the historical control group, Micra patients had 54 percent fewer hospitalizations and 87 percent fewer system revisions.
One-tenth the size of a conventional pacemaker, Micra is a capsule-shaped, leadless pacemaker that can be inserted through the femoral vein. The device has flexible tines that attach to the interior of the right ventricle, and these tines can be disengaged to reposition or retrieve the device, if needed. The device was awarded CE Mark in April 2015 and currently is an investigational device in the United States. The first successful U.S. implantation of the Micra device was in early 2014. Medtronic hopes to gain FDA approval by next year.
Manufacturers are developing less invasive, miniature pacemaking device technologies to eventually replace conventional pacemaking systems, which are associated with more complications, such as infection and broken leads.
"Pacemaker leads, which connect the chest-wall generator of a pacemaker to the pacing electrode in the heart, are the “Achilles’ heel” of pacing and defibrillation systems. Over time, they wear out, which often necessitates their risky removal and replacement," Mark S. Link, M.D., writes in an editorial in the NEJM. "A self-contained leadless pacemaker that can be placed directly into the heart is an appealing prospect."
Dr. Link notes that Medtronic's Micra and St. Jude Medical's Nanostim miniaturized pacemakers "appear to be remarkably similar" and have demonstrated similar primary safety and efficacy profiles in separate studies. However, he adds, "Whether the long-term results will show that these devices remain safe and effective over time and that these leadless devices are as durable as transvenous pacemakers remains to be seen."
Since both Micra and Nanostim deliver single-chamber ventricular pacing, these devices are limited for patients with atrial fibrillation or bradycardia, and, in their current iterations, would provide no benefit for the majority of pacemaker recipients, which include those with sinus-node dysfunction, heart block, or heart failure patients who need left-ventricular resynchronization, according to the editorial.
