Navigating The Universe Of Risk In Medical Device Development (With Infographic)
By Steve McPhilliamy, Partner, Insight Product Development
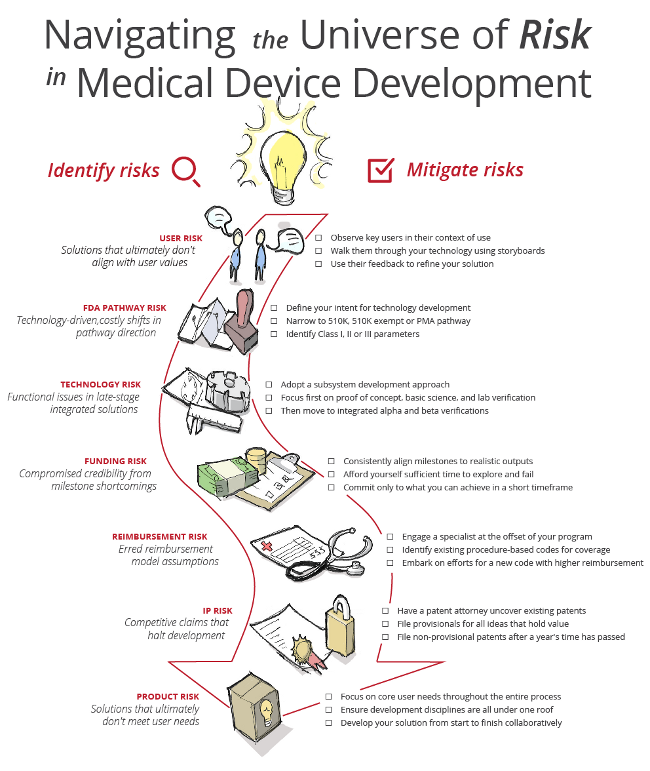
Varying levels of risk exist within any development process, but these risks are compounded in the development of products and services for the medical device space. To successfully navigate this development environment, a strong understanding of healthcare-specific processes and the inherent risks at each stage of development are critical. Failing to recognize these risks can lead to time-intensive and costly missteps.
More than 25 years of medical development work — for companies ranging in size from startups to Fortune 500 corporations — has given us deep understanding and significant experience in navigating the device development process. While many of the risks we’ll outline are from the perspective of a startup, they are relevant to all device development projects and should prove helpful in identifying risks, mitigating issues, decreasing time-to-market, and increasing the likelihood of product success.
Risk identification and mitigation strategies are mapped out in the infographic below, and then explained in detail afterward.
User Risk
At the center of every successful technology is a solid understanding of the value it holds for the user. Surprisingly, some companies that have spent years fully developing sophisticated technologies discover, in the end, that the technology has no clear market or value to core users. By leveraging research methods to understand key stakeholders’ hierarchy of value, you can drive technology development that fits market needs before it goes too far down an interesting — but unsuccessful — technology path.
A broad range of research methods exist to confirm whether your solution aligns with users’ core needs. These methods answer a variety of questions, including whether the solution fits users’ lifestyles and whether the solution can be easily understood and adopted. To that end, the research includes observational techniques within the environment of use, interview techniques that pose open-ended questions for deeper discovery into user choice and preference, and scenario-of-use storyboard reviews that explore new process improvements and technology projections. These methods allow target users to identify new use patterns, reveal a solution’s design shortcomings, and ultimately align development direction to focus on value.
FDA Pathway Risk
Defining a clear and actionable plan for development that aligns to the shortest regulatory pathway can have a huge impact on the success of a medical device. Development programs that change direction in favor of interesting technology ideas can quickly shift a streamlined 510(k) pathway to a complex PMA pathway, jeopardizing a program’s timeline to market launch. Beyond costing the company untold time delays, this risk can also tax the energy and efforts of a development team, diverting them to activities not aligned to the near-term technology solution.
Successfully navigating the regulatory path requires a clear sense of intent for technology development alignment from the outset of a program. Whether you’re pursuing 510(k) exempt, 510(k), or PMA clearance for a Class I, II or III device, clearly establishing your direction and plan at the beginning of development, and then measuring and documenting your progress consistently against that initial direction, is the best strategy for mitigating FDA pathway risk.
Technology Risk
A key risk in developing solutions that incorporate several integrated components is technology risk. Discovering that functional issues exist in your product solution, effectively rendering it inoperable at the end of your development program, can be a particularly costly proposition, based on the substantial time required to identify exactly what part of the solution failed. To circumvent this risk, developers can adopt a subsystem development approach, affording them the opportunity to rapidly identify what works and what doesn’t along the entire development path.
This approach moves development along in discreet, finite steps that focus on basic science, proof of concept, bench-top and lab demo verification of the individual components before moving on to integrated alpha and beta iterations. This progressive approach is not only a more reliable method to mitigate performance risk, but ultimately a more cost-effective one.
Funding Risk
Another risk is over-promising technology capabilities early in the development process, which can set the entire development effort up for potential failure and loss of funding. Project funding is built on trust. When key financial stakeholders in a project can see progress that is aligned with or surpasses their expectations, trust grows. To mitigate funding risk, development programs must align milestones to realistic and achievable outputs.
This does not mean avoiding innovative solutions in favor of the easiest technology embodiment; it means affording yourself sufficient time to explore and fail. Commit only to what you can ensure that you will be able to successfully achieve in an efficient timeframe. Doing this consistently will demonstrate the pattern every financial stakeholder wants to see, structure a series of repeatable successes, and lead to the best chance for initial and continued funding.
Reimbursement Risk
An equally perilous risk is unknowingly moving down a device development pathway without either an associated reimbursement code or a clear plan to lobby for a new reimbursement code. Without a distinct plan of attack in either instance, the likelihood is high that your solution simply won’t be adopted by the healthcare system. Whatever the company size, it behooves all medtech developers to perform due diligence up front with the guidance of industry reimbursement specialists.
Such specialist can either help you identify procedure-based codes that already exist and could cover the cost of using your device, or help you embark on the years-long American Medical Association (AMA) and government lobby effort for a new code that yields higher percentage reimbursement rates for your solution. Not engaging in this due diligence, and forging ahead blindly on the belief that a code surely exists in the current reimbursement model, could easily delay your market launch by years.
Intellectual Property (IP) Risk
Another substantial development risk is having the proverbial rug ripped out from under you by competitive IP, effectively preventing you from continuing to develop your technology. To best guard against these competitive threats and position yourself to claim the IP your company is developing, it’s imperative to first understand the space you want to own.
The best first step is to engage a patent attorney in the earliest stages of your development planning, allowing you to uncover existing patents and identify what remains fair game. As your development vision is articulated and developed iteratively, a patent attorney can file provisional patents to protect your ideas — even if they are not yet fully formed. For many startups, IP represents the company’s primary value. If that IP is not well-managed or strategically structured, company value can decrease based on the increased risk of competitor IP claims and ownership.
Product Risk
The broadest and farthest-reaching of all development risks is product risk. This risk stems from focusing too narrowly on any one element in development, losing sight of core user needs and value in the end. This can be a common occurrence in environments that do not engage in collaboration, throughout the entire development process, to best inform the final functional design.
For example, consider an engineer tasked with developing a functional prototype using marketing product requirements alone. Absent collaboration with a human factors professional and an industrial designer, the independent development decisions of the engineer may not include elements like enhanced usability or visual appeal to end users. Development that occurs in silo — without researchers, designers, human factors experts, and engineers informing every design and functional iteration — can lead to misalignment with user value.
The best defense against product risk is undertaking every development project, from beginning to end, with a collaborative, multidiscipline team under the same roof.
About The Author
Steve McPhilliamy is a partner at Insight Product Development, as well as executive director of Insight’s Accelerator Labs, an accelerator program distinctly focused on health tech startups. Steve draws on more than 20 years of design experience, having established the first accelerator program in the Midwest to advance meaningful technology toward commercialization, grow the Chicago innovation ecosystem, and improve lives. He holds a baccalaureate in industrial design from the University of Kansas. Contact Steve at smcphilliamy@insightpd.com or visit www.insightpd.com