New Autofluorescence Device Tracks Wound Infections
By Chuck Seegert, Ph.D.
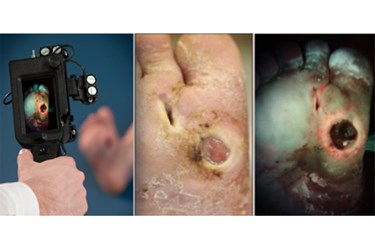
The clinical evaluation of chronic wounds has long suffered from subjective bias. A new device, however, may make invisible bacteria detectable without lab tests or other interventions.
Wound infection is often diagnosed by clinical judgment, a subjective and somewhat inaccurate method. Augmenting this with bacterial cultures to establish an infection level is more effective, but is generally slow and can require days to get results. Combinations of these approaches with their inherent drawbacks can lead to the use of the wrong antibiotics and the potential development of resistant strains of bacteria.
Given the relatively undeveloped state of diagnostic medicine in this area, a team from Princess Margaret Cancer Centre and Techna Institute for the Advancement of Technology for Health, University Health Network (Toronto) decided to approach the problem head on with a device they’ve named PRODIGI, according to a recent article from MedGadget.com.
In the article, the device’s inventor, Dr. Ralph DaCosta, describes PRODIGI as a “novel fluorescence-based imaging platform combining low-cost instrumentation and specific autofluorescence identification for in situ, real-time wound infection assessment, therapeutic guidance, and response monitoring.”
The PRODIGI system was tested in a research study published by the team in the SPIE Journal of Biomedical Optics. A key feature that makes the device especially attractive is its ability to work using a phenomenon called autofluorescence. Autoflourescence allows the device to detect bacteria using the inherent properties of the bacteria instead of applying some sort of contrast agent. Just the presence of the bacteria is enough for PRODIGI to work.
As part of the study, the team used the bioluminescent Staphylococcus aureus bacteria to validate the effectiveness of PRODIGI in a nude mouse model. They were able to do this by examining the level of fluorescence and correlating it with bacterial load, thus showing the accuracy of the device at detecting infection.
According to the article, Dr. DaCosta’s team is moving forward with clinical investigations, as PRODIGI has been approved as a class II device by Health Canada. Clinical trials are ongoing at the University Health Network and commercialization of PRODIGI is being pursued by Dr. DaCosta through a company called MolecuLight Inc.
Understanding wound infections is a concern on many fronts including the military. Recently the Department of Defense disclosed a device based on molecular probes for wound infection diagnosis. This technology was able to detect infections that lab cultured samples had missed.
Image Credit: DaCosta et al.
