New Self-Assembling Nanofibers May Impact Tissue Engineering, Drug Delivery
By Chuck Seegert, Ph.D.
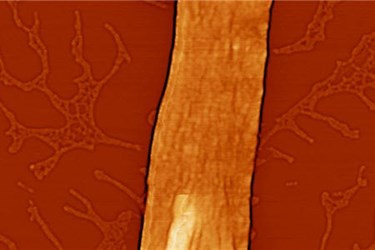
Self-assembling nanostructures are nothing new, but researchers at Carnegie Mellon University have discovered a novel way of organizing protein molecules into long chains that are similar to naturally occurring cellular filaments.
Composed of modified green fluorescent proteins (GFPs) bound to flexible linker molecules, these nanofibers may have application in tissue engineering and drug delivery.
"We have demonstrated that, by adding flexible linkers to protein molecules, we can form completely new types of aggregates. These aggregates can act as a structural material to which you can attach different payloads, such as drugs. In nature, this protein isn't close to being a structural material," said Tomasz Kowalewski, professor of chemistry in Carnegie Mellon's Mellon College of Science, in a recent press release.
GFP molecules typically dimerize, or bond together in pairs, at hydrophobic patches on the protein, but that is the extent of the natural process. The Carnegie Mellon team, however, used a process called click chemistry to add dialkyne poly(ethylene oxide) (PEO) to the GFP molecules, while leaving the natural dimerization site free. The GFP’s propensity to link via natural hydrophobic patches combined with the PEO-dialkyne linkers enabled the fibrous assembly in this unique system.
"Our protein-polymer system gives us an atomically precise, very well-defined nanoscale building object onto which we can attach different handles in very precisely defined positions. It can be used in a way that wasn't ever intended by biology," Kowalewski said. Payloads, as described by Dr. Kowalewski, may be specific drugs, or other molecules that are bonded on to the GFP molecules.
Results of the research study showed structural fibers formed in this new system of GFPs and flexible linker molecules may extend for tens of microns, while maintaining uniform widths that are less than 100 nanometers. When exposed to fluorescent light, the GFPs were luminescent, indicating that the 3D structure was intact and the function of the proteins had not been appreciably impacted by the chemical treatments.
Additionally, the nanofiber could be disassembled by applying ultrasonic energy, and then would naturally reassemble after a couple of days. According to the press release, this attribute of “reversible fibrous self-assembly” is highly desirable for tissue engineering, drug delivery, nanoreactors, and imaging applications.
A multi-disciplinary team worked closely to study the nanofibers from a biological standpoint, as well as a polymerization chemistry standpoint. Morphology of the structures was studied using confocal microscopy and atomic force microscopy, while the bonding behavior was modeled by a computer with a technique called dissipative particle dynamics. With this data, it became clear that the assembly behavior was likely driven by the hydrophobic patches on the GFP molecules.
"This was purely curiosity-driven and serendipity-driven work," Kowalewski said in the press release. "But where controlled polymerization and organic chemistry meet biology, interesting things can happen."
Research interest in nanoscale technologies continues to escalate. Applications are wide-ranging and include advancements in regenerative medicine, diagnostics and drug delivery.
Image Credit: Angewandte Chemie International Edition
