Novel device delivers drugs via the bladder
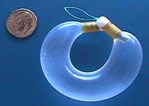
Situs is in Phase 1-2 clinical trials testing UROS Infusor delivery of I-OXY (oxybutynin), a new formulation of a drug that is currently given orally for the treatment of overactive bladder. This disorder affects an estimated 17 million Americans. More than 50 percent of the people seeking treatment for overactive bladder cannot tolerate the side effects of oxybutynin or other orally administered drugs currently used as therapeutic treatments.
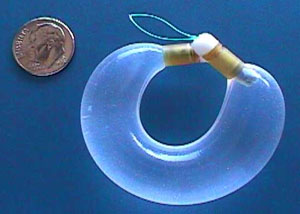
"Using the UROS Infusor we can deliver a special formulation of oxybutynin, called I-OXY, directly into the bladder over an extended period of time," said Rodney A. Appell, M.D., Head, Section of Voiding Dysfunction and Female Urology at the Cleveland Clinic Foundation. "With this novel delivery method we lessen oxybutynin's side effect profile and provide patients with a potentially more effective treatment for overactive bladder. The initial clinical data from the Phase 1-2 trial supports what was previously demonstrated in animal studies, specifically that intravesical delivery requires less drug to achieve similar blood levels as compared to oral administration and that there is a steady uptake of the drug over 28 days."
Situs is a combination specialty pharmaceutical and drug-delivery device manufacturer pioneering an intravesical drug-delivery platform.
Edited by Ursula Jones
Managing Editor, Medical Design Online
