Populating The UDI Database – A Look At GUDID
By Dan O'Leary, President, Ombu Enterprises

A fundamental purpose of unique device identification (UDI) is to allow users to learn specific device characteristics. The manufacturer puts a device identifier (DI) on the device label that corresponds to a DI record the manufacturer loaded into a database. Anybody with the DI can enter it into the database and retrieve the public information from the DI record that identifies the device. Figure 1 shows how this process works.
The database is the Global Unique Device Identification Database, or GUDID. It is usually pronounced either “goo did” or “good ID.” The FDA’s Center for Devices and Radiological Health (CDRH) maintains the database, but the device manufacturer loads the information and updates it as necessary.
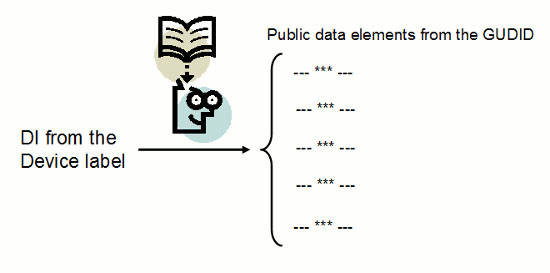
Figure 1: Using the DI to obtain information
There are some important caveats about the information in the database:
- It provides device identification information but no information on tracking or tracing.
- While FDA calls it a “global” database, the information is specific to devices marketed in the U.S. For example, if the manufacturer doesn’t list the device with FDA, it is not eligible for the database.
From the manufacturer’s point of view, there is a process to follow. It involves setting up an account, loading the DI records, and updating the DI record for changes.
Creating An Account
Before loading DI records, you need to set up an account with CDRH. You can obtain the form to request an account by completing a form at the Request a GUDID Account page on the FDA website.
Before you can complete the account request form, you need to get some information and make some decisions:
- Determine which part of your business will be responsible for the DI records. In a multisite business, each business unit could maintain its own DI records, or the company could consolidate the responsibility at one location.
- CDRH manages the GUDID accounts by linking them to your company’s DUNS (Data Universal Numbering System) Number. The DUNS number is a unique site-specific nine-digit identification number provided by Dun & Bradstreet (D&B). The entity responsible for maintaining the DI records must have a DUNS number.
- Make sure the name and address associated to the DUNS number is correct. You will not enter your company information in the DI record, only the DUNS number. The DI record will retrieve the information by looking up your DUNS number.
- Identify the people in your organization who will have specific GUDID roles as shown in Figure 2. These include the regulatory contact, any coordinators, and any label data entry (LDE) Users. For each person, you will provide the first name, last name, email address, telephone number, physical location, and associated DUNS number.
- Choose the submission options (described below) you will use.
- Determine if you will use a third party to enter information on your behalf. If so, you need the third party’s DUNS number.
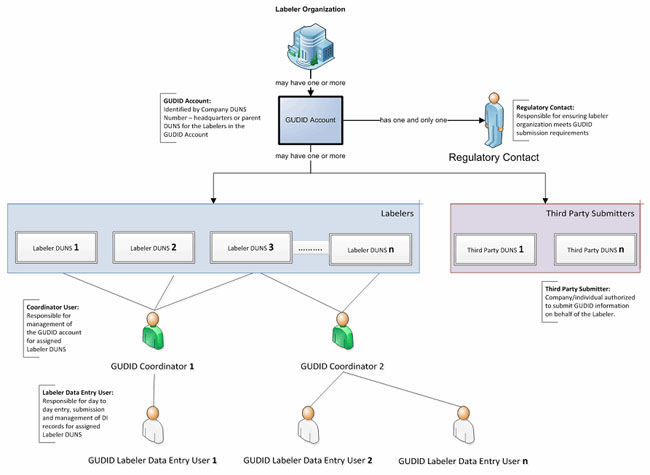
Figure 2: The organizational structure to support GUDID (click to enlarge)
Gathering The Data
In order to load the data into a GUDID DI record, you must gather it. For many companies, this will be a large effort, because the data is probably not in one place. Start with a list of all the devices you sell. Then, identify their standard packaging configurations. For example, you might sell the device individually. Your catalog could also include a box of two, a case of five, and a carton of 10. These are all standard packaging configurations.
CDRH has a list of the information you need to provide for each device. You can download it as an Excel file from the Global UDI Database (GUDID) page on the FDA website. Figure 3 shows the data elements.
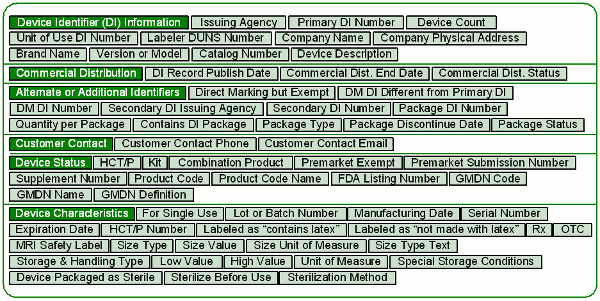
Figure 3: GUDID data elements
Data Submission Methods
There are two methods to populate the DI record, the GUDID web interface and HL7 SPL (Structured Product Label). In the web interface, an LDE user can enter data directly. HL7 SPL uses an XML file to populate the DI record.
When you request a GUDID account, you specify which method you will use. You can use both methods, and for the HL7 SPL option, you will need both methods. In addition, HL7 SPL users will need additional accounts for FDA’s electronic gateway and run test scripts. This can take two to three months. You can download the HL7 SPL implementation files from the Global UDI Database (GUDID) page on the FDA website.
DI Record Life Cycle
The DI record has a lifecycle that will eventually make it available to the public.
A draft DI record is a work in progress. It provides an opportunity to develop the record and work on it over time. This is a temporary state, so GUDID deletes the record after 180 calendar days of inactivity. However, any activity resets the timer.
DI records can be in draft only when using the web interface. HL7 SPL submissions cannot be draft DI records. The record submitted must be complete and correct when using HL7 SPL. Before submitting an HL7 SPL record, the manufacturer has an opportunity to develop it offline.
A draft DI record must pass the business rules before submission to GUDID. After submission, it is either unpublished or published, depending on dates entered in the record. An unpublished record means the publish date is in the future (i.e., publish date > today). A published record means the publish date is today or in the past (i.e., publish date ≤ today).
After the publish date, there is an additional grace period of seven calendar days starting the day after the publication of the DI record. During this period, you can edit anything in the record except the publish date. After the grace period, the editing capabilities are very limited.
Setting The Publish Date
Set the date in the future, to be sure you have time for review and edits. If you set the publish date = today, the DI record will be available to the public immediately. You should set the publish date about seven days before the compliance date for existing products or the first shipment date for new products. This helps ensure you have a correct record available to the public when your device ships.
Commercial Distribution Status
If you discontinue a device, you need to update the DI record by entering a commercial distribution end date. GUDID has a commercial distribution status field. Before the commercial distribution end date, the field is set to “in commercial distribution”. On or after the commercial distribution end date it switches to “not in commercial distribution”.
There are similar provisions for packaging configurations.
It is very important to realize that a published DI record never goes away. The GUDID saves it indefinitely and allows public access. The device could be available to users long after the manufacturer stopped selling it.
