Proper Care And Handling Of Coupons Used For Swab And Rinse Recovery Studies For Total Organic Carbon Analysis
By Jayen Diyora, Max Chen, Sarthak Pandey, and Andrew Walsh

Part Of The Cleaning Validation For The 21st Century Series
Surrogate surfaces, or “coupons” as they are commonly called, have been widely used for many years to simulate pharmaceutical manufacturing surfaces or medical devices for the performance of swab and rinse recovery studies. Today, these coupons are also used for cleanability testing, disinfectant testing, and visual inspection qualification programs. Although such coupons have been in use for many years there has been little to no available information on how these coupons should be manufactured, handled, prepared, or maintained. This article will discuss the proper care and handling of these coupons for swab and rinse recovery studies, in particular for total organic carbon (TOC) analysis.
Coupons For Recovery Studies
Coupons are typically square to rectangular surfaces with a 5-cm x 5-cm or 10-cm x 10-cm test area (see Figure 1 for an example). In the past, coupons were made only from stainless steel, but they can be manufactured from any of the various materials of construction (MoCs) that are used in the equipment used by pharmaceutical and medical device industries. The 2015 update to the European Medicines Agency’s Annex 15 (Section 10.12) requires recovery studies for all product contact materials in the equipment and with all the sampling methods used.1 Due to this requirement, there is an increasing demand for these coupons made from many different MoCs. Today, suppliers can produce almost all of these MoCs, with up to 40 different MoCs now available.2
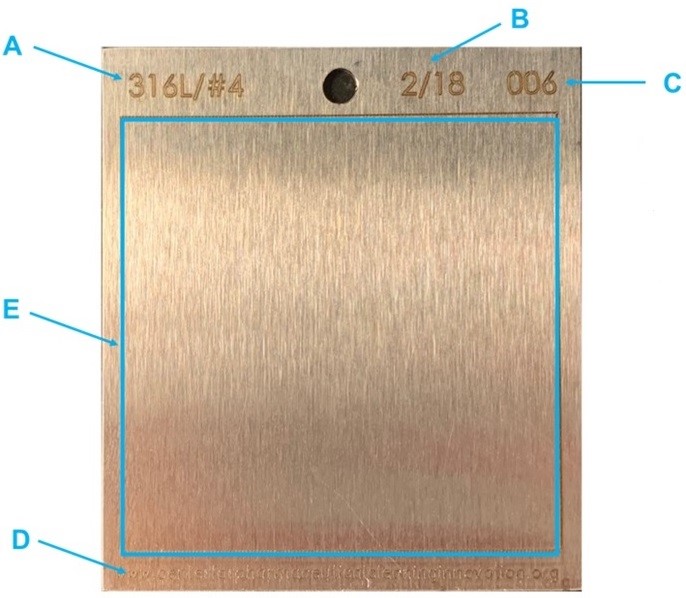
Figure 1: Example Coupon — This coupon is manufactured from 316L stainless steel with a #4 finish and engraved with (A) the MoC, (B) the date of manufacture, (C) the coupon number, and (D) the identification of the manufacturer. The test area (E) is inscribed and in this example is a 5-cm X 5-cm area. This coupon is compliant with ASTM Standard G121.3
Successful and reliable results for recovery studies require that the coupons used must be properly manufactured in the first place, and then they must also be carefully handled, stored, and, in particular, appropriately cleaned before use.
Cleaning Of Coupons
Unfortunately, there is currently no available guidance on the cleaning of coupons prior to use in swab or rinse recovery studies, although there is some minimal guidance for cleanability studies and visual inspection studies.
In 2018, an existing ASTM standard was updated to include pharmaceutical and medical device manufacturing in its scope.3 This standard, ASTM G121 Standard Practice for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents, was originally developed by the National Aeronautics and Space Administration (NASA) for use in oxygen systems.4 This standard has detailed information on the preparation of coupons for testing but only some minimal information on the cleaning of coupons for use in pharmaceutical cleaning studies.
G121 simply states:
“Test coupons should be cleaned after use with a defined procedure that restores the test coupons’ surfaces to their original state.”
Another ASTM standard,5 written specifically for pharmaceuticals and medical devices, E3263 Standard Practice for Qualification of Visual Inspection of Pharmaceutical Manufacturing Equipment and Medical Devices for Residues, also simply states:
“5.7.2 Surrogate surfaces shall be thoroughly cleaned and examined before preparation to ensure the surrogate surfaces are representative of the worst case for the manufacturing equipment or device surfaces and relevant for the qualification.”
The need for cleaning of coupons used in swab recovery studies was emphasized in a 2017 article by Novartis,6 where they determined that: “The lack of a well-defined procedure that consistently cleaned coupon surface was identified as the major contributor to low and variable recoveries”. The Novartis article described two multistep procedures using an ultrasonicator and multiple cleaning agents to clean coupons (Table 1).
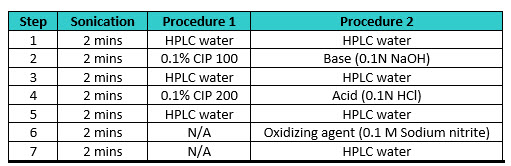
Table 1: Coupon Cleaning Procedures as per Novartis Article6
Like Novartis, one of the areas of research for the Center for Pharmaceutical Cleaning Innovation (CPCI) laboratory has been reducing the variability of swab and rinse recovery study results. For example, recoveries of greater than 100%, while theoretically impossible, are commonly found with easy-to-recover compounds whose recoveries are close to 100%. This is typically explained as due to slight variations between the results of the standards versus the swab or rinse samples. However, this cannot account for results equal to or greater than 110%, which are known to happen in particular with TOC studies. Coupons that have not been completely cleaned can have residues that will contribute to the overall TOC results and generate results greater than 110%. It should be quickly understood that if a positive bias such as this can occur at the 100% recovery level, then a TOC recovery result of 80% might actually be only around 70% (Table 2). It should also be realized that, in these cases, this problem would probably never be noticed or even suspected.
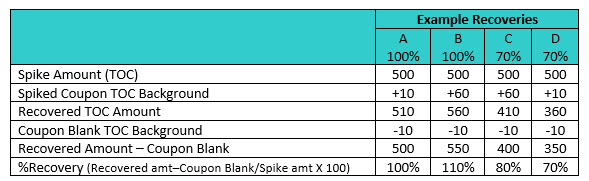
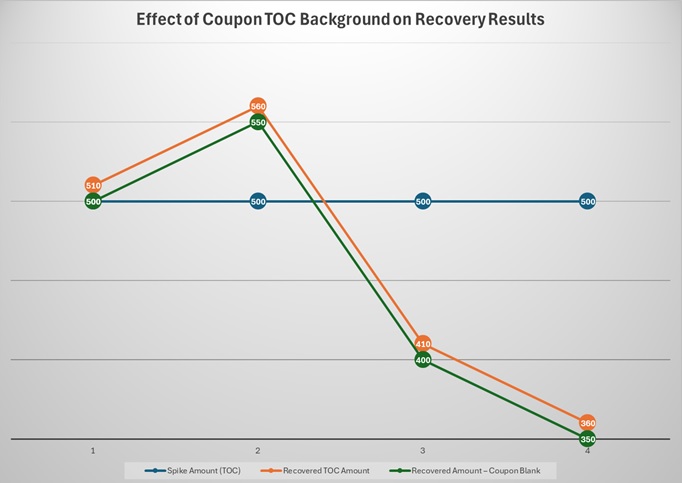
Table 2: Effect of Coupon TOC Background on Recovery Results —
A 100% — The Spiked Coupon TOC Background (10ppb) is equal to the Coupon Blank TOC (10ppb) and does not interfere with the recovery, which is 100%.
B 100% — The Spiked Coupon TOC Background is 50 ppb higher than the Coupon Blank TOC and interferes with the recovery, which is 110%.
C 70% — The Spiked Coupon TOC Background is 50 ppb higher than the Coupon Blank TOC and interferes with the recovery, resulting in an 80% recovery.
D 70% — The Spiked Coupon TOC Background is equal to the Coupon Blank TOC and does not interfere with the recovery, which is actually 70%.
Research at CPCI has already identified that swabs can be a source of variability in recovery studies (same as in Table 2) due to variable TOC levels of the swabs and has developed a cleaning procedure for swabs to reduce this background variability. Similarly, the coupons used in these studies also can contribute to this background variability. Since a “coupon blank” is used to adjust the results of the swab samples in the study, any organic carbon residues on these coupons could bias the recovery results, either positively or negatively. It is therefore important that all the coupons used in these studies have very low to no TOC background noise. This problem can be minimized by scrupulous cleaning of the coupons before use.
One issue with cleaning these coupons is that typical cleaning agents normally contain a significant amount of organic carbon compounds that can leave organic carbon residues on the coupons that you are trying to clean. To address this problem, CPCI recommends using a coupon cleaning agent that does not contain any organic carbon.
An example of such a cleaner, such as one developed at CPCI, would be a combination of a very mild abrasive (calcium carbonate) with a powerful cleaning agent (trisodium polyphosphate). Neither of these compounds contains organic carbon. Consequently, this cleaning agent could both physically and chemically remove all residues from the coupons and leave no traces of organic carbon. This formulation provides very low coupon blank results, resulting in more accurate and reliable TOC swab/rinse recovery study results. The cleaning procedure for this cleaner is also very quick and simple (Figure 2 below).

1. Pipette 0.5 mL of low TOC water to the surface of the coupon.

2. Apply about 0.5 gram of CPCI Coupon Cleaning Agent onto the coupon.

3. Firmly rub the test area of the coupon in back-and-forth and up-and-down patterns with a non-shedding wipe.

4. Finally, rinse the coupon with low TOC water to ensure any residues from tap water are rinsed off. (Note: Coupons may be rinsed first with tap water but must be thoroughly rinsed with low TOC water afterward, as tap water is high in TOC.)
Figure 2: CPCI Coupon Cleaning Procedure — This cleaning procedure takes only 10 minutes to clean five coupons, the number of coupons typically used in a swab/rinse recovery study. This procedure has been used successfully with coupons of 316L stainless steel, borosilicate glass, and PTFE and should be successful with many other MoCs.
Cleaning Procedure Comparison Study
A study was performed comparing Novartis Procedure 1 to the CPCI coupon cleaning procedure. Twenty visibly dirty coupons were prepared to make four sets of five coupons (Figure 3).

Figure 3: Example of Five Dirty Coupons prepared for Comparison Studies
Five of the coupons in one set were swabbed and analyzed for TOC without any cleaning. Two other sets of five coupons were then cleaned by two analysts using the CPCI developed coupon cleaner. The final set of five coupons was cleaned following Novartis Procedure 1. All swab samples were analyzed using the Beckman Coulter QbD1200+ Laboratory TOC Analyzer. The results are shown in Table 2.
The TOC results for the visibly dirty coupons shown in Figure 3 revealed a range from 4,892 ppb to 8,459 ppb. All four sets of the dirty coupons prepared were expected to be within this range prior to cleaning the coupons using the CPCI procedure and Novartis Procedure 1. While coupons after recovery studies are never as visibly dirty as those used in these studies, these coupons were prepared to be “extra dirty” to challenge the effectiveness of these two cleaning procedures for evaluating their cleaning performance. The results of these studies are shown in Table 3.
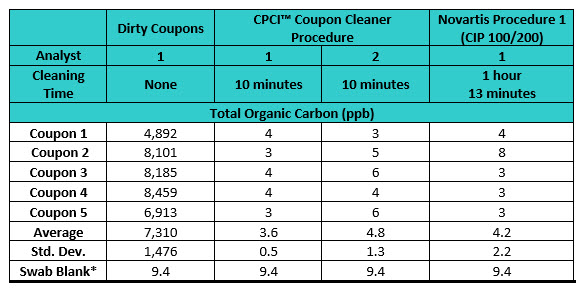
Table 3: Total Organic Carbon Results from the Comparison Studies. *All data adjusted for swab blank (swab blank contained three swabs). Note: CPCI has developed and uses a swab recovery procedure that uses three swabs to obtain the highest recoveries.
All swab samples were analyzed using the Beckman Coulter QbD1200+ Laboratory TOC Analyzer.7
These results were compared by ANOVA (analysis of variance) using statistical software. The interval plot at the 99% confidence interval from the ANOVA study is shown in Figure 4.
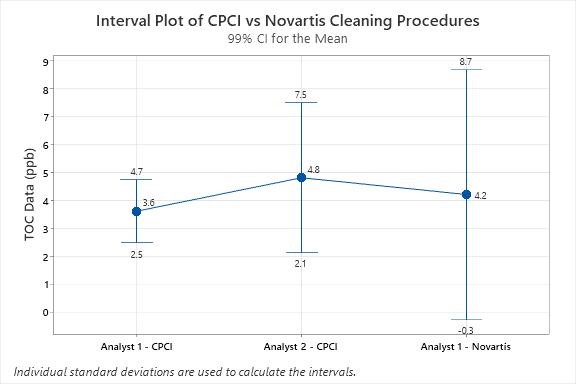
Figure 4: Interval Plot of Residual TOC Results after Applying the CPCI vs. the Novartis Cleaning Procedures — In this study Analyst 1 was an experienced laboratory subject matter expert and Analyst 2 was an engineering intern inexperienced with the cleaning procedure. Analyst 2 had slightly higher results and variability than Analyst 1. Analyst 1 also showed slightly higher results and wider variability for the Novartis procedure. While this statistical analysis indicates a higher variability from the Novartis procedure vs. the CPCI procedure, at this low level of residue (parts per billion) this difference can be considered of no practical significance
Both the CPCI and Novartis cleaning procedures resulted in very low residual TOC results. The residual TOC levels obtained after cleaning the coupons with CPCI cleaning procedure ranged from 3 to 6 ppb, whereas the Novartis cleaning procedure resulted in residual TOC levels in the range of 3 to 8 ppb. The differences observed in residual TOC levels could be due to variable amounts of organic carbon applied when preparing “dirty” coupons. However, regardless of the cleaning procedure and initial amounts applied, both cleaning procedures resulted in very low residual TOC levels, with an overall mean of 4.2ppb (overall standard deviation = 1.47ppb) and the difference can be considered insignificant. The Novartis article provided no data on residues after their cleaning procedure and only the effect on recovery studies. so no comparison could be made between this study and the Novartis study.
Although both the CPCI cleaning procedure and Novartis Procedure 1 left minimal residues on the coupons, the CPCI cleaning procedure is significantly easier, faster (taking only 10 to 15 minutes to clean five coupons), does not require an ultrasonicator, and is much less expensive to perform, whereas cleaning coupons using Novartis Procedure 1 took more than an hour to clean five coupons and used expensive cleaning agents that need to be prepared. Preparation of the cleaning agents was not included in the time to clean for the Novartis procedure.
The cleaning of coupons is an essential part of TOC swab or rinse recovery studies to minimize interference from TOC residues left over from a previous product or cleaning agent. Using the CPCI cleaning procedure will not only avoid interference of TOC during recovery studies but will save significant time for routine cleaning and maintenance of the coupons used in the recovery studies.
Handling Of Coupons
Based on studies in the laboratory, CPCI has developed a number of good practices that it recommends should be followed when handling coupons during TOC recovery studies.
- Wear gloves (cleaned) during sample handling to prevent inadvertent contamination through fingerprints that can lead to biased recovery results.
- Rinse with low TOC water prior to handling coupons to reduce the risk of contamination that can lead to biased recovery results.
- Avoid the use of isopropyl alcohol or any other organic solvent for cleaning coupons for TOC studies as these may leave interfering residues.
- Inspect coupons for defects (e.g., scratches) that may interfere with the recovery study prior to use. If a coupon is found to be defective it should be replaced.
- Refrain from talking during recovery studies or during validation sampling to prevent small drops of saliva from contaminating the coupons or the samples. This will lead to falsely high TOC values.
- Wear a mask during recovery studies or during validation sampling may further minimize the risk of sample contamination.
Storage Of Coupons
Proper care of coupons will provide many years of successful and reliable use from the coupons. Coupons should be stored in protective containers to prevent damage to, or contamination of, the coupons. Coupons should be engraved with the material of construction for easy confirmation of the correct coupons prior to performing a study and stored in a similarly labeled container. The date of manufacture should also be engraved on the coupon, which can be used in an inspection program for defects in the coupons through their ongoing use.
Summary
With increasing regulatory requirements and scrutiny of the recovery studies performed for cleaning validation it is becoming more and more important to focus greater care on the cleaning of test coupons and their maintenance. Using a coupon cleaning procedure, such as the one developed by CPCI, will not only avoid interference of TOC in the recovery study analysis that can significantly bias the results but also save significant time in the cleaning and maintenance of these coupons.
Peer Review
The authors wish to thank Ralph Basile, Sarra Boujelben, Alfredo Canhoto, Ph.D., Gabriela Cruz, Ph.D., Mallory DeGennaro, Parth Desai, Kenneth Farrugia, Ioanna-Maria Gerostathes, Ioana Gheorghiev, MD, Igor Gorsky, Ovais Mohammad, Mariann Neverovitch, Miquel Romero Obon, Laurence O'Leary, Ajay Kumar Raghuwanshi, Kailash Rathi, Osamu Shirokizawa, Stephen Spiegelberg, Ph.D., and Basundhara Sthapit, Ph.D., for reviewing this article and for providing insightful comments and helpful suggestions.
References
- EudraLex Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use Annex 15: Qualification and Validation
- https://www.centerforpharmaceuticalcleaninginnovation.org/store/c3/All_Coupons_come_in_a_3D_Printed_Storage_Box.html
- ASTM G121 Standard Practice for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents
- Song, Ruijin and Andrew Walsh, ASTM Standards For Cleanability Testing Of Pharmaceuticals And Medical Devices, Pharmaceutical Online, September 2020
- ASTM E3263-22e01 Standard Practice for Qualification of Visual Inspection of Pharmaceutical Manufacturing Equipment and Medical Devices for Residues, https://www.astm.org/e3263-22e01.html
- Imad A. Haidar Ahmad, James Tam, Xue Li, William Duffield, Thomas Tarara, Andrei Blasko Cleaning verification: Exploring the effect of the cleanliness of stainless steel surface on sample recovery, Journal of Pharmaceutical and Biomedical Analysis 134 (2017) 108–115
- https://www.beckman.com/toc-analyzers/qbd1200-plus
