Novartis Partners with Robot Pill Maker To Pursue "Holy Grail of Drug Delivery"
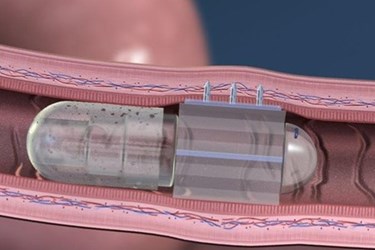
Startup Rani Therapeutics has announced a collaboration with Novartis to investigate Rani’s novel drug delivery platform, a tiny "robotic pill." The two companies hope to achieve delivery of large-molecule biologics like insulin, a monumental task the researchers call the “holy grail” of drug delivery.
Under the terms of the agreement, Rani will conduct feasibility studies over the next 18-24 months using Novartis’ line of proprietary biologics. At the conclusion of the studies, Novartis will have the option to license Rani’s technology. According to a press release, Rani is pursuing similar partnerships with other developers of biologic drugs.
“The delivery of large molecules orally is considered the holy grail of drug delivery, and there have been many failed attempts before us. We understand the magnitude of the problem we are pursuing and we are confident that our approach has the potential to radically change the way biologics are administered to patients,” said Mir Imran, CEO of Rani, in the release.
Rani’s proposed design is a capsule that consists of two chemical compartments filled with citric acid and sodium bicarbonate, respectively. As the pill dissolves in the digestive system, barriers between the two substances erode, allowing them to mix and create a chemical reaction that pushes micro-needles of sugar through the outer layer of the capsule and into the lining of the small intestine. The sugar needles can be filled with drugs that are delivered into nearby blood vessels as the sugar is absorbed. Because the small intestines do not contain nerve endings, the patient feels nothing during delivery.
Reuters reports that the technology could be used to treat a number of conditions, including diabetes, rheumatoid arthritis, and multiple sclerosis — all of which require daily painful injections. If successful, the robotic “needle pill” could revolutionize patient comfort and convenience.
Last year, Massachusetts Institute of Technology (MIT) researchers unveiled the design of a similar device, potentially capable of delivering large molecules like insulin. Rather than sugar, their “needle pill” used stainless steel that was gradually exposed as pH levels in the digestive system wore off the capsule’s outer layer. So far, the technology has only been tested in pigs.
In their study published in the Journal of Pharmaceutical Sciences, the MIT researchers noted that doctors and patients both preferred oral medications over injectables, because they are easier for patients to manage and the molecules are distributed more evenly throughout the body.
For the past several years, Novartis has been making strategic investments in the growing field of wearable and ingestible technology. In 2012, Proteus Digital Health, a company in which Novartis invested in 2010, announced that the FDA had cleared its ingestible sensor for tracking patient compliance. More recently, Novartis licensed Google’s contact lens technology that can monitor glucose levels.
In the press release, Rani's Imran expressed enthusiasm over Novartis’ interest and support. “We are committed to delivering on our promise to radically transform how biologic drugs are delivered to millions of patients, and this financing is an important step forward.”
