Redefining Reliability In Medical Electronic Devices
By Ravi Subrahmanyan, Fred Sporon-Fiedler, Anthony Primavera, and Danbo Shen
A smart, end-to-end solution to the product lifecycle yields devices that patients can count on to work right, every time.
Reliability is mandatory for medical electronics used in monitoring and therapeutic applications due to lack of redundancy, miniaturization and functional integration. Conventional approaches to reliability can have limitations due to lack of historic data typical of new technologies, low signal to noise ratio related to low production volumes and long product lifetimes.
MSEI has deployed a comprehensive, systematic, end-to-end advanced reliability methodology (e2ARM) that focuses on the application of reliability technologies throughout a device’s lifecycle - from development and manufacture to monitoring. Leveraging over 35 years presence in the medical electronics industry, the scalable solutions for design margins analysis, highly automated physical and digital connected factory has a proven track record for over 3 million electronics assemblies.
Medical electronic devices are used for a wide variety of applications, ranging from monitoring and therapy to life-sustaining functions. A monitoring device such as a pill camera, for example, is swallowed by the patient and has a lifetime of just a few hours, while the majority of life critical or life-sustaining devices - some of which are implanted into the human body - have to last anywhere from a few years to decades. A cochlear implant may be implanted into a baby and is expected to work for the entire lifespan of the patient, for example 80+ years. Likewise, an implanted cardiac rhythm device such as a pacemaker or defibrillator might remain in the human body for anywhere between 5 and 15 years.
Regardless of how long medical electronic devices might need to operate, assessing and assuring their reliability is essential in both monitoring and therapeutic environments. When a device fails, the patient may be seriously impacted either through improper diagnostics or insufficient therapy. Miniaturization and functional integration almost always preclude the use of redundancy in these designs. At the same time, a device replacement is often not an option, for example, in implanted devices where the surgical procedure can increase the risk of infections. And therein lays the crux of the problem; reliability in these devices must be assured; however, for the reasons above, this gets more challenging every day.
Limitations of Conventional Reliability Approaches
Reliability is the probability that a system or part will perform its required function under stated conditions for a specified period of time. It is often determined using reliability metrics like mean-time-between-failures (MTBF), defect rate per year (ppm/year) or failure-in-time (FIT).
In the medical electronics industry, there are no common, industry-wide accepted practices for assessing and assuring a device’s reliability. Often military or space methods are employed, something commonly referred to as the “shake, rattle and roll” approach, whereby the various parts of a device are tested to prove that they are acceptable. As an example, parts (e.g., components or modules) are subject to stress conditions during the development cycle. As long as they meet specified stress conditions, the design process moves on. During production, the parts can also undergo as-built electrical or functional testing and stress screens to ensure performance within specified limits.
The tests conducted assess reliability using a combination of stochastic and mechanistic methods, many of which stem from standards like IPC, MIL and others. The end result is a bathtub curve, as shown in Figure 1, where a device’s failure rate is plotted as a function of time. Typically, the failure rate is characterized by three phases: 1) an early life or infant mortality region where the failure rate decreases as a function of time, 2) a constant failure rate region and 3) a wear-out region where the failure rate increases as a function of time. Early life failures are generally due to anomalous damage stemming from deviations in workmanship, while those in the constant failure rate region are attributable to a variety of stochastic variations in materials and processes. Failures in the wear-out region are due to intrinsic, time-dependent propagation of damage caused by a variety of mechanisms like corrosion, migration and fatigue.

With these tests, a device’s failure modes are accelerated by temperature and voltage, as well as environmental and mechanical stresses, among other things. They precipitate failures and can accelerate certain infant mortality and wear-out failure modes. During the product lifecycle, engineers often focus on eliminating the impact of wear-out mechanisms in the design phase and addressing early life failures using a technique like burn-in. Oftentimes they use methodologies that are deduced from other applications and optimized for use with high reliability medical electronics. Unfortunately, the applicability of these methodologies is often limited due to a number of issues.
The first issue is that these approaches aren’t always compatible with new technology. For example, implantable medical electronics are highly customized and miniaturized for use in the human body. They achieve low power, high functionality and their small size by utilizing new technologies. Conventional approaches may not accelerate the mechanisms that produce failures for such new technologies in their use environment and therefore, can be limited in their scope.
The signal-to-noise ratio - from a production quantity point-of-view, that is — is another issue. Imagine you wanted to produce 10-million cell phones a year with a defect rate of 1 ppm/year. Because of the large quantity of cell phones, there would be an ample amount to test. In contrast, the entire implantable medical industry might have only 2-2.5 million devices in total, so there is not a large enough quantity to detect small variations. In other words, there is no real data to compare to and therefore, the signal is extremely small compared to the noise.
Another issue is that many of the life-sustaining medical electronics devices have very long lifetimes and this make prognostic establishment of long reliability challenging. Further complicating matters, reliability in implantable medical electronic devices is not something that can be attained through redundant circuitry; there is just no room for built-in redundancy. Likewise, the power constraints of these devices preclude redundancy.
Charting a New Path In Reliability Assessment and Assurance
What’s clear is that a new reliability approach is needed and must be an inherent part of the device’s design, manufacture and post-production use. This need is all the more acute today, as market conditions like continued electronic industry consolidations and the transition to new components and materials further stress the limitations of conventional reliability approaches.
Through decades of amassed experience in the medical industry, Micro Systems Engineering (MSEI), a subsidiary of Micro Systems Technologies (MST), has developed a systematic, end-to-end advanced reliability methodology (e2ARM) that focuses on the application of reliability technologies throughout a device’s lifecycle - from development and manufacture to monitoring. The methodology has three primary goals: to ensure a robust design, contain any of the design’s anomalous behavior and continually monitor and contain random failures.
The MSEI methodology treats reliability not as a point solution, but rather a comprehensive one that covers all phases of a device’s lifecycle. That process begins before the device is designed and follows it through to the device’s end of life with 24/7 monitoring of its performance and the continual harvesting and collection of data in real time. That data, which at minimum is in the terabytes per year range, provides for a complete view of how well the design, manufacturing, components and product is performing. What makes this all work is near 100% factory automation, control during manufacture to ensure any anomalous device behavior is prevented and the use of a broad range of methods, equipment, and processes during all three phases of the device’s life cycle. Figure 2 outlines the e2ARM framework containing the development, manufacturing and monitoring elements.
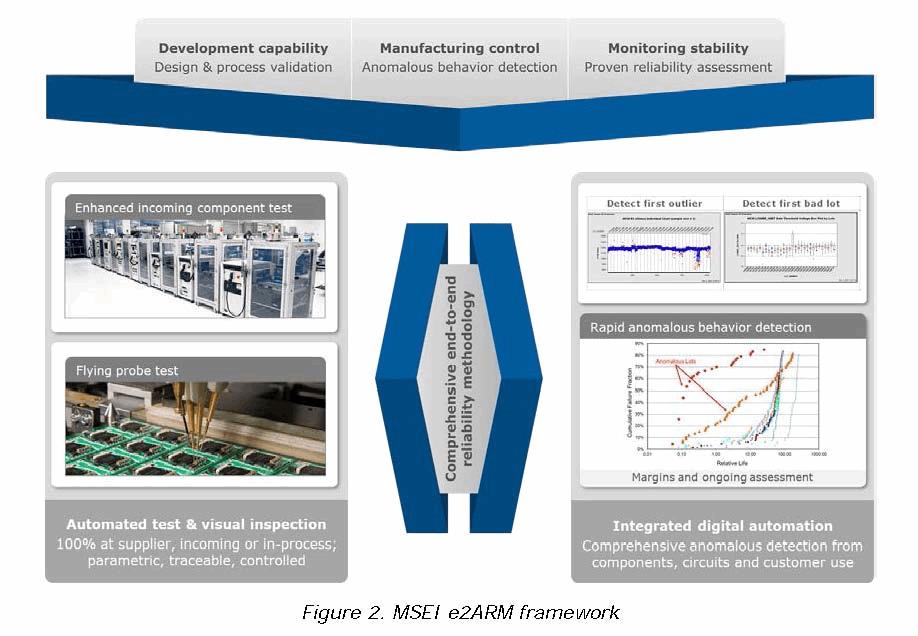
- Development: During development, the period of time prior to the release of a device to serial production, the MSEI methodology focuses on improving the product’s design margin by eliminating failure modes. To accomplish this goal, a risk assessment and margins analyses are performed on each new, incoming component, processes and designs. A stress profile to precipitate latent defects, a characterized test methodology to detect precipitated defects and a sampling plan based on the sensitivity of the expected change and allowed error are used to verify requirements are met. The residual risks following verification & validation are mitigated using comprehensive manufacturing controls and monitoring approach.
- Manufacturing: During manufacturing, the time when a released design is serially produced under controlled and qualified conditions, the MSEI methodology focuses on detecting and containing anomalous device behavior. To accomplish this goal, MSEI first defines all programs/recipes for automation, including automated visual inspection and electrical test. Next, 100% testing of the product is performed to assure margins and acceptance. Finally, any anomalous behavior encountered is contained. It is not used until its root cause has been established and fixed.
- Monitoring: During monitoring (the end use of the product), the MSEI methodology focuses on obtaining a quantified basis for random failure modes through reliability monitoring. With this monitoring, performed at MSEI’s digital and physical factory, the device’s stability is continuously assessed to determine and then validate its reliability (Figure 3). Burn-in and Lot-based stress testing to precipitate latent defects are also performed. In addition, the closed-loop improvement activity begun during the design phase is continued, along with monitoring for any anomalous behavior and ongoing assessments of parametric analyses to assure long-term reliability.

Key Advantages of Reliability Redefined
What makes MSEI’s reliability methodology unique is that it succeeds where more conventional approaches fall short. Its continual monitoring of devices while in use means that both real-time and predictive modeling is possible; a key factor in ensuring future device generations will be better and more reliable. This also makes the methodology applicable to a wide variety of medical electronic components, materials, modules, and technologies.
Another advantage is that unlike conventional approaches, MSEI’s methodology has high sensitivity to anomalies (i.e., a very low signal to noise ratio) because of the highly controlled automated and digitally connected factory. This enables automated, serialized monitoring, data collection and trend interpretation.
Finally, the MSEI methodology resolves the challenge of a long product lifecycle, by mechanistic use case modeling, margins assessments and stability monitoring.
Uniquely Suited to Address Reliability Challenges
Reliability is critical for medical electronic devices — not only those used for life-sustaining purposes, but also for other monitoring and therapeutic applications. MSEI’s unique and innovative reliability methodology (e2ARM) addresses the capability development, control and monitoring challenge head on. Leveraging over 35 years’ experience in the industry, 80% of the staff are focused on suppliers, components, process, and product development. The emphasis on automated physical and digital factory infrastructure and strong parametric monitoring/control of quality from incoming to customer has a proven track record for over three million electronic assemblies. At the end of the day, that level of involvement translates into better, more reliable devices that patients can count on to work right, every time.
About the Authors
Dr. Ravi Subrahmanyan is the Executive Director, Advanced Technologies, Micro Systems Engineering, Inc. (Lake Oswego, OR). He leads a team for selection and deployment of design and manufacturing technologies for use in high reliability microelectronics applications.
Fred Sporon-Fiedler is Director of Future Technologies, Micro Systems Engineering, Inc. His work for the past 9 years spans component, technology and process development for MSEI products. Prior to MSEI, Fred spent the bulk of his career in Semiconductor process development and RF component development/reliability with HP, Agilent and Avago Technologies.
Dr. Tony Primavera is Director of Process Technologies, Micro Systems Engineering, Inc. For the last 8 years at MSEI, his group has been focused on new technology, production capabilities and manufacturing infrastructure. Tony spent many years in the electronics industry developing process technology and automated assembly systems.
Danbo Shen is Manager of Component Technologies, Micro Systems Engineering, Inc. For the past 14 years, he has been working on high performance and high reliability component development. Prior to MSEI, Danbo worked at IBM in design and development large scale computer systems.
Click here for the PDF version of this article.
