Reviewing FY2017 FDA 483s: Training Failures Or A Learning Paradigm?
By Troy Fugate, Compliance Insight

I find it interesting to evaluate the FDA 483 data, year after year, and see the same pattern of non-compliance. An interesting response can be observed at conferences when this data is presented: Everyone begins to frantically write down the numbers. I know — I used to do the same thing. Running back to the office to show everyone the “data.” Thinking it would somehow impact their thinking on “quality.” It never did. The analysis of data is key to making decisions. In this FDA regulated industry, we must act on data or we are simply stumbling around in the dark, hoping to have an impact.
But how do we act on the data of previous 483s? What do we do with that data? Similar to the annual product reviews, we gather data on FDA observations, makes graphs, and then … file it away. But there is an alternative! Let’s go through the data first and then discuss what should be done with it.
The Data
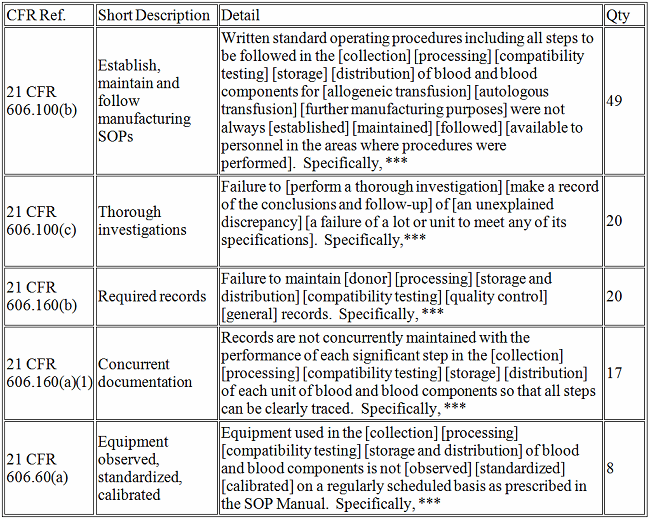
The fiscal year for the FDA extended from Oct. 1, 2016 through Sept., 30 2017, during which time the FDA issued 5,155 observations. The following table breaks down the top five observations pursuant to the issuing center (listed largest to smallest in number of observations):
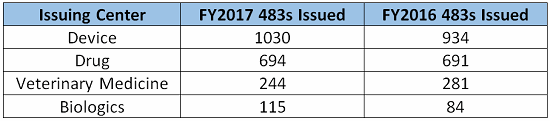
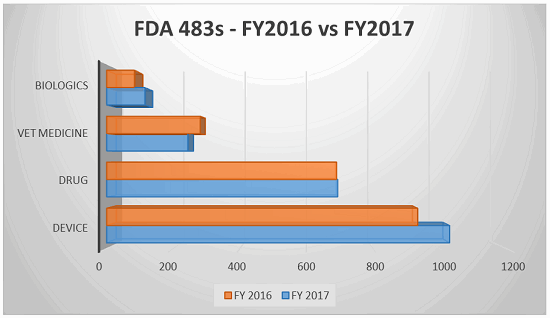
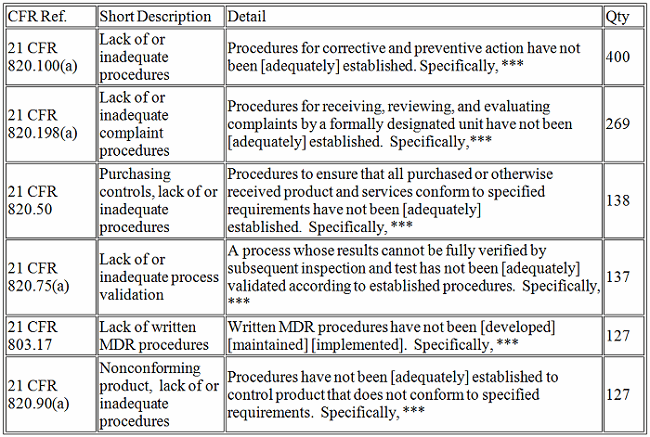
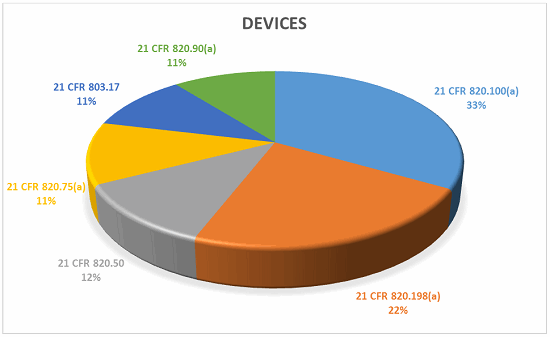
The top five CFR observations in 2017 per center are provided below.
Devices
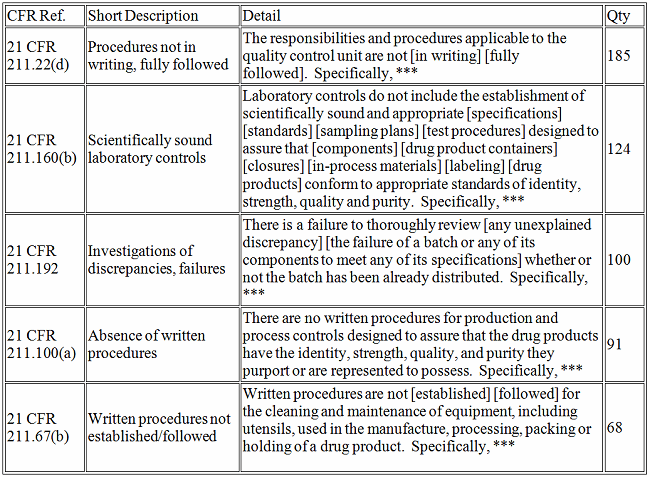
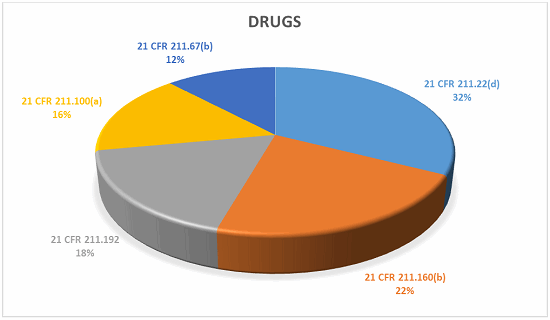
Drugs
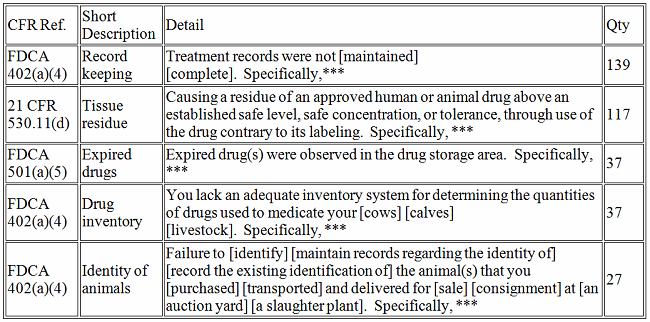
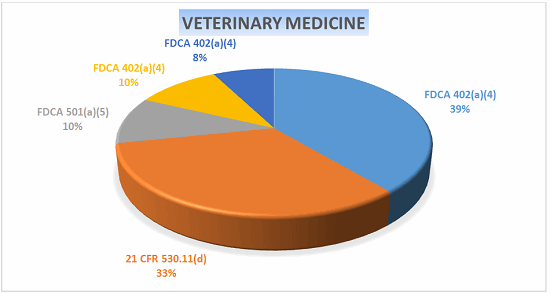
Veterinary Medicine
Biologics
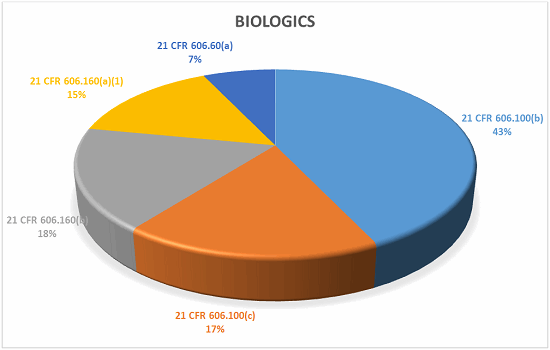
What does it all mean?
Now that we see the data on observations, what should we do with them? Are we just compiling data to make neat charts? To tell people “See — we can’t do that”?
A Background Story
Looking over the failures of the firm, the executives were frustrated and exhausted. The CEO turned to me and asked: “Why do our people go through training but still fail to do what they are supposed to do.” The programs and techniques were in place but the outcomes were the same. My reply: “Interesting question — and key to the issues that we are seeing. To implement true change in habit, we have to think about how people learn and forget.”
The Astonishing Statistics
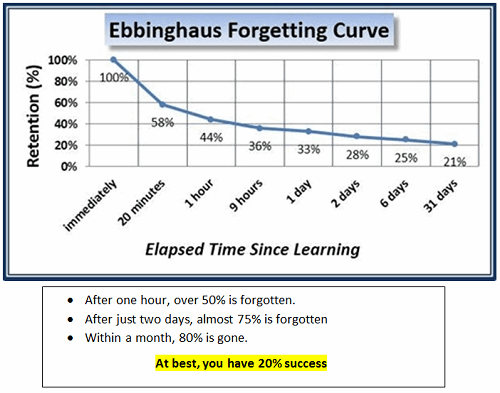
If you want to experience this phenomenon, ask yourself or others in your group these questions:
- Who provided GMP training at your firm last year, and what was the topic?
- What was improved or changed because of the training?
It was a critical discovery of a flaw that we, as an industry, perpetuate: Annual or periodic GMP training expected to change habits was not much more than a “checking the box” compliance event. And so, we keep doing it repeatedly … yet expect different results.
Successful training goes beyond the typical classroom approach. You must repeat the message in a meaningful way over an extended period of time. And that’s not all…
Why Don’t They Do It?
You can’t tell me that people are not aware of the requirements in the GMPs. Look at the observations presented here for the past two years: not following procedures, not having procedures, inadequate validation, etc. Who would think these practices (no procedures, not following procedures) are acceptable? Answer: No one! People just don’t put the GMPs into their own work habits, or they don’t realize that they are doing it incorrectly.
To understand how to change work habits, we must understand how people think. A great book on the subject — “Why Employees Don’t Do What They’re Supposed To Do” by Ferdinand Fournies — provides some useful insight. I’ve summarized some of the book’s points below, along with some of my own insight:
- They don’t know or understand why they should do it.
- They don’t know how to do it.
- They don’t know what they are supposed to do.
- They think your way won’t work.
- They think their way is better.
- They think something else is more important.
- There is no positive consequence for doing it the right way.
- They think their way is doing it the right way.
- There is/may be a negative consequence for doing it the right way.
- There are obstacles beyond their control.
- They have personal limitations.
- There is no way to do it that way.
- They have an engrained habit to do it another way.
Summary
Having the data is great. However, we must act upon what it is telling us. Right now, it is saying that we are doing the same things wrong, year after year. We can improve, and we must. It just takes time to mentor and develop the workforce to resolve the root causes of these observations.
About The Author:
 Troy Fugate, VP of operations at Compliance Insight, utilizes 30 years of hands-on insight into quality assurance, validation, workforce development, and training skills to evolve GMPs from a regulation to a value-added habit. He has been a partner in Compliance Insight since 2001, helping companies around the world, and serves in a variety of roles including workforce development strategic compliance initiatives, and FDA mitigation
Troy Fugate, VP of operations at Compliance Insight, utilizes 30 years of hands-on insight into quality assurance, validation, workforce development, and training skills to evolve GMPs from a regulation to a value-added habit. He has been a partner in Compliance Insight since 2001, helping companies around the world, and serves in a variety of roles including workforce development strategic compliance initiatives, and FDA mitigation
Fugate has been a proponent of proactive, sustainable compliance solutions and is an enthusiastic supporter (not to mention co-leader) of Good Supply Practices initiated by Xavier Health. You can contact him at tfugate@compliance-insight.com or connect with him on LinkedIn.
