3 Rules For Designing Medical Device Tests
By Lynessa Erler, Director of Product Testing & Test Systems, MPR Associates
With the development of any medical device, the greatest concern is always to protect the patient from injury. However, device development managers are challenged with how to most effectively allocate resources among design, test, quality, and production groups to achieve this goal. Our experience has shown that with proper integration of testing into the development process of a device, you can effectively manage product failure rate and keep development costs low.
1. Understand The Product Ecosystem
The best time to start thinking about testing your device is at the very early stages of the project. As you develop the specifications for the device, you need to also identify the test needs for the device. By managing the development of your test tools as part of the device’s ecosystem, you can ensure that your test tools meet your test requirements at all stages of development. Integrated project teams that include test engineers can specify the requirements for the test tools, layout the schedule and budget for their development, and treat the test tools like a project (like a subsystem of the device).
Being able to break a project up into smaller bits that can be tested early — even before being integrated into the final device — allows early detection and correction of design deficiencies. Identify points in the device development schedule where design engineers will have need for specific test tools and develop a test tool schedule that provides the appropriate tools at these milestones. All too often we hear how difficult it is to get input from the design team: They are just too busy to discuss how the device will be tested. By working with the design team’s schedule and giving them access to tools that they can use to inform design, however, they begin to value the integral role that testing can play and allocate time to give their input.
When developing the test tool schedule, consider how the information from the test will be used when planning test tool validation activities. Test tools used in verification testing require validation, but if these same tools are used to provide data for critical design decisions, earlier validation of the tool is warranted.
2. Understand The Test Tool Lifecycle
Imagine spending thousands of dollars developing a fully automated inspection system for production testing of your device, only to discover that your device continually fails the inspection. What if upon probing further you discover that the test system is not at fault, it is just more effective at detecting the defect? We often see the test tools used during design, verification, and production developed and maintained by separate divisions without regard for the test needs of the other organizations.
By managing the lifecycle of your test tools as part of the device’s ecosystem, you can ensure that your test tools meet your test requirements at all stages of development. This maximizes the potential for reuse of equipment and software. Access to high-quality, rigorous test tools also allows the early detection of design defects. The requirements of the test tools that we use evolve as the device’s lifecycle progresses.
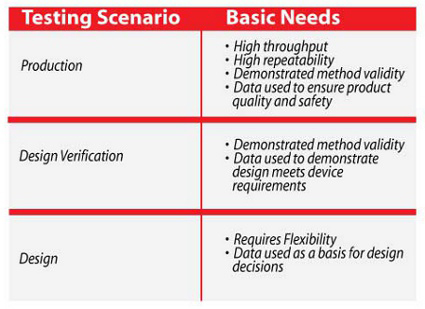
One of the first steps to effectively managing testing is to properly identify the kinds of testing required. This helps to determine how the specific goals and constraints for these test scenarios differ. Medical devices are primarily tested in three scenarios: testing to support design development, testing to demonstrate that the design meets the device requirements, and testing to demonstrate that the final production meets the requirements.
In the design stage, tools need increased flexibility so that testing can quickly answer questions such as: What are the failure modes and how much margin is there? Even though this information may never get passed out of the design group, important decisions are based upon it. Therefore, the integrity of this data is no less critical. Errors at this stage can have far-reaching effects. In the verification stage, the scope of testing has become more specific and the test tools and results receive more scrutiny. This is typically the first stage at which test tools are validated. In production, the priority of test tools shifts to maintaining test integrity with high throughput.
Often the test methods employed in verification and production have their roots in the test tools used in the design stage, but they are not validated until later use.
The selection of an easily scalable test platform is one very effective tool in managing the changing test tool requirements. Scalable platforms provide the hardware, drivers, and toolsets to allow test code to run on a variety of hardware platforms with minimal rework required. For example, an analysis algorithm may be developed to run on a lower channel count test system to support design. However, with a scalable platform, this code can also run on a high channel count, higher speed system used in production testing. With a scalable platform, time spent early in the project to develop and validate accurate and reliable test tools can be leveraged later in the test tool lifecycle.
3. Train Test Engineers
A test engineer plays an extremely important role in the project. The right test personnel with the right experience and skills are critical to the success of every device development project. The test engineer is involved from the beginning, providing input on everything from design documentation (such as requirement specifications) to the deployment of test systems for manufacturing. Moreover, the test engineer develops experience over time and can draw on lessons learned from previous projects to help accelerate testing on future projects.
One of the best investments your company can make is to establish a center of excellence (COE) for testing and test systems. This center of excellence can help reduce the costs of testing and increase the overall quality of your products by implementing a few measures. First, the COE can save a whole repository of validated test tools that can be reused within and across projects. The COE can also be in charge of managing test equipment and increasing their utilization. Further, the COE can take the responsibility for establishing best practices and enforcing appropriate testing management. The COE drives the development of testing expertise and efficiency across all aspects of product development projects.
By integrating knowledgeable experienced test engineers into the design team in the early stages, testing requirements are identified and addressed earlier. This allows the design group to have access to quality, rigorous test tools. This facilitates the identification of design problems earlier in the development lifecycle, where correction is less expensive. This also allows planning of the test tool lifecycle, maximizing reuse, and reducing the overall cost of test.
About The Author
Lynessa Erler is the director of testing and test systems in MPR’s Product Development group. Since joining MPR in 1994, she has become a subject matter expert and provides technical leadership in verification of medical and diagnostic device design, management, and verification of software development, digital signal processing, and data acquisition and control. Ms. Erler is also MPR’s senior National Instruments/LabVIEW expert and has applied this expertise in the development of products and test systems.
