Updated 3Shape Implant Studio Receives FDA 510(k) Clearance
Copenhagen – 3Shape announces that the U.S. Food and Drug Administration (FDA) has granted (510k) USA market clearance for the latest version of its Implant Studio software, which features an edentulous patient treatment workflow.
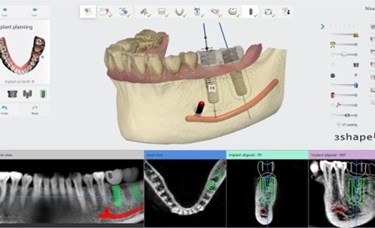
3Shape Implant Studio software merges CT/ Cone Beam CT imaging with 3D digital surface scans of the teeth and gingival situation to provide a digital view of dental patients. This enables dental professionals to evaluate the clinical situation, including, bone density and nerve positions, for creating prosthetic implant planning and surgical guide design.
Implant Studio introduces a dedicated workflow for implant planning and surgical guide design for edentulous patients. Edentulous patients can now be treated by using only CBCT/CT scan data and through the application of a dual scan protocol. The latest version of the software also features more than 20 new functional improvements including, the ability to manual align scans, gingiva-supported surgical guides and an innovative two-piece guide design. In addition, surgical guides designed in Implant Studio are optimized for milling and/or 3D printing.
“3Shape is very excited to receive FDA (510k) market clearance once again for our Implant Studio software. Importantly, the newest version has now added an edentulous patient workflow. This is extremely valuable as people of all ages are choosing dental implants to meet their restorative needs. Implant Studio provides dental professionals with an easier-to-use and more efficient workflow for caring for their patients,” says Flemming Thorup, 3Shape, president and CEO.
The dental implant market in the U.S. is projected to reach $5 billion by 2018. With 3 million Americans already having implants. That number is predicted to grow by 500,000 per year, according to the American Academy of Implant Dentistry.
Implant Studio is available through 3Shape resellers. Availability to end-users will depend on the specific system configuration.
3Shape experts will be demonstrating Implant Studio including complete workflows using intraoral and CBCT scans at the 2016 Midwinter Meeting in Chicago, February 25-27.
About 3Shape
3Shape creates 3D scanning and CAD/CAM software solutions. Our precision Quality Control solutions enable manufacturers to reduce production cycles, streamline efficiency and as a result, increase profitability. A privately-owned company, 3Shape has nearly 700 employees with a product-development force of more than 230 professionals. Offices and service centers located in the Americas, Asia and Europe serve customers in more than 100 countries. Company headquarters are in Copenhagen, Denmark.
Source: 3Shape
