Shockwave Medical Secures $45 Million Series C Funding For Lithoplasty Device
By Jof Enriquez,
Follow me on Twitter @jofenriq
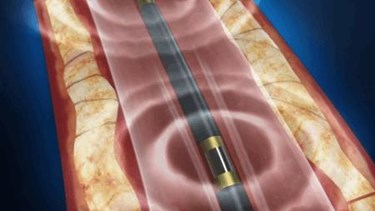
Shockwave Medical says it has raised $45 million in Series C financing to further development of its Lithoplasty ultrasound device, expanding into new therapeutic areas via additional clinical trials and commercializing the product in the United States and the European Union markets.
Lithoplasty combines lithotripsy – a procedure using shock waves to break up kidney stones – with balloon catheter angioplasty to prop open blocked arteries. Shockwave Medical's device emits intermittent sound waves that target and disrupt calcified plaques, which then require only an integrated low-pressure balloon to dilate the blockage and restore blood flow in the narrowed artery.
FDA approved in September, the device will be available for limited commercial release in 2017 in the U.S., where nearly nine million people have peripheral artery disease (PAD), according to the company.
“Lithoplasty is poised to be a paradigm-changing technology for the treatment of advanced cardiovascular disease. This financing will enable the company to continue taking the steps necessary to ensure the technology reaches its full potential,” said Shockwave Medical CEO and co-founder Daniel Hawkins in a news release.
FDA clearance was granted based on positive results of the single-arm, multi-center study conducted in two phases: Safety and Performance Study of the Shockwave Lithoplasty System (DISRUPT-PAD) and Shockwave Lithoplasty DISRUPT Trial for PAD (DISRUPT PAD 2).
The company says it is readying another trial called DISRUPT PAD III, which is said to be the largest ever multi-center randomized study designed to exclusively enroll patients with calcified PAD. The 300+ patient study will test the device in conjunction with drug-coated balloons, providing physicians with "foundational Level I evidence to guide therapy" in this difficult-to-treat patient population.
Besides having been tested among PAD patients, the Lithoplasty device has been evaluated in patients with calcified coronary artery disease (CAD) prior to drug-eluting stent (DES) implantation through DISRUPT CAD, a pre-market, prospective, multi-center, single-arm study conducted at seven centers in Europe and Australia. Results from this study showed the device's ability to dilate calcified coronary lesions safely, with few complications.
“When you consider the treatment challenges created by calcified lesions, it is clear there is a large market opportunity for Lithoplasty. The strong clinical results generated using a device built on a balloon-based platform offer a unique and compelling alternative to currently available therapies,” said Michael Sjöström, co-founder and Chief Investment Officer, Sectoral Asset Management, as well as lead investor of this Series C financing round.
Other participants in this round were mutual funds advised by T. Rowe Price Associates, Inc. and returning investors, led by Sofinnova Partners, Venrock, RA Capital, Deerfield, and Ally Bridge Group.
Founded in 2010 and based in Fremont, Calif., Shockwave Medical has raised $102 million in venture capital funding to date, according to PitchBook.
