Stomping Out Diabetic Foot Ulcers

By Bob Marshall, Chief Editor, Med Device Online
Would you be willing to step on a device that looks like a bathroom scale each morning, for 20 seconds, if doing so could help you retain mobility, and perhaps even save your life? A diabetic foot ulcer (DFU) is an open sore/wound occurring in approximately 15 percent of patients with diabetes, according to the American Podiatric Medicine Association (APMA). The APMA also estimates that 14 to 24 percent of diabetic patients developing a foot ulcer will require an amputation. However, research has shown it doesn’t have to be that way; the development of foot ulcers can be prevented. How so, you ask? According to Podimetrics, the answer is stepping on its SmartMat for 20 seconds each day.
Helping Diabetics Put Their Best Foot Forward
I talked with Podimetrics CEO and co-founder, Jonathan Bloom, M.D., to learn more. He explained:
“To use the Podimetrics system, a patient stands on the SmartMat for 20 seconds per day in their home. Temperature data collected from the patient’s feet are sent to the cloud, where the Podimetrics system analyzes the data using proprietary algorithms. The software looks for hotspots, or areas of inflammation, which are otherwise undetectable. It has been well established that these hotspots strongly correlate with the eventual development of foot ulcers. If a developing hotspot is detected, the healthcare provider is notified based on their custom protocol and an intervention is planned. Most interventions require a patient to stay off his/her feet while the foot heals itself.”
Clinical Data Gives Podimetrics A Leg Up On The Competition
The Podimetrics remote temperature monitoring system received FDA 510(k) clearance in October of 2015, but — having seen other companies develop technology in this space, only to fall short of consumer adoption — Bloom and his team realized clinical data would be necessary for the SmartMat to succeed. Bloom realized he would have to consider SmartMat’s commercialization from the earliest stages of the project.
“In order for doctors to prescribe it and health plans to pay for it, there must be data to show the device can actually diagnose a DFU prior to its manifestation, AND it must show that patients will use it regularly, and that it will be easy for them to use,” Bloom said.
Last month, Podimetrics announced results from a recently completed clinical study that included 129 subjects with diabetes who had experienced prior DFU, and was conducted in a 34-week, multi-center format. In the study, the Podimetrics remote temperature monitoring system correctly identified 97 percent of observed DFU with an average lead time of 37 days. In addition, 86 percent of the study subjects used the SmartMat at least three times per week, and 88 percent of respondents reported it being easy to use.
Making Sure Users Don’t Lose A Step
I asked Bloom about the potential for user “fatigue” over the long haul: how will Podimetrics ensure users remain engaged and committed to using their SmartMats? He explained, “the technology enables patients to be monitored, and they can be contacted if use decreases to reinforce the need to continue to use the system on a regular basis.”
We also discussed the possibility of building a community aspect into the Podimetrics system, enabling users to connect with one another, encourage each other to use the device regularly, and share their experiences. Podimetrics has a unique opportunity in this area, since the company’s clinical study was conducted at VA medical centers. There probably is not a more cohesive group of study subjects than U.S. military veterans. They are a community that volunteered to face adversity together in entering the service, and could be counted on to band together again to support one another in their battle against DFU. I’d be willing to bet Bloom already has his team looking into the feasibility of adding a community feature to the system.
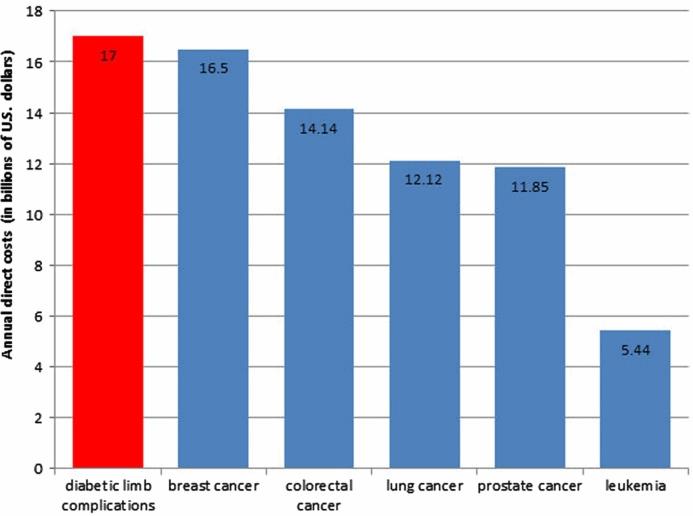
Walking Back The Cost Of Diabetic Limb Complication Care
The annual cost for the treatment of diabetic limb complications is significant. The chart below compares the costs of diabetic limb complications with five predominant forms of cancer.
The system of care for the diabetic foot: Objectives, outcomes, and opportunities (PDF Download Available). Available from: https://www.researchgate.net/publication/257840246_The_system_of_care_for_the_diabetic_foot_Objectives_outcomes_and_opportunities [accessed Jul 20, 2017]
It seems the potential for health care cost savings related to the early detection and treatment of DFU could be significant. I asked Bloom to speculate on how much of a dent Podimetrics might put into the annual $17 billion cost associated with DFU, and while he laughed politely and reiterated his enthusiasm for the benefits of the SmartMat, he didn’t offer a dollar value or percentage reduction number. I pushed a little more, but he clung to his positive — yet nondescript — answer, offering only that he expects his device will help to reduce cost via early detection, leading to more effective treatment overall. I teased him that if this medical device CEO gig doesn’t work out for him, given his answers to my questions, he may have a future in politics…
Taking The Market In Stride
I have spoken with a lot of early-stage medtech leaders over the past several decades, and I was impressed by Bloom’s approach and demeanor. From the beginning of our discussion, his enthusiasm and passion for the DFU space and the patients was palpable. Bloom is obviously excited about what Podimetrics is accomplishing and his attitude is upbeat and positive. What was even more impressive to me, though, was that Bloom exhibits no tendencies toward haste or rash judgment.
With FDA clearance in late 2015 and positive data from the recent clinical study, most startup CEOs would be planning a global launch. I might add that many of those same startup CEOs would subsequently strain relationships within their own organization, fail to deliver product on time (losing customer goodwill), perhaps have inordinately high initial complaint rates, or even need to recall product, inviting regulatory scrutiny.
Thus, I asked Bloom about potential competition and the path ahead. “There does not seem to be much in the way of competition, at least not anyone with positive clinical data like ours. We have our systems in 650 homes right now and will continue to focus on our VA users for the next 12 months. We are working on some pilot usage of the system in private healthcare plans to gain a better understanding of that market, but for now, we are focused on adoption. To us, adoption is everything!”
