6 Technology Trends You Can't Afford To Ignore (In Your Medical Device Design)
By Jim Pomager, Executive Editor

When it comes to purchasing medical technology, today’s healthcare provider is looking for wireless-capable devices that will help them fill electronic health records (EHRs), support agile workflows, and meet outcome- and value-driven healthcare mandates. If you don’t shift your design thinking to meet these needs, prepare to start losing business. You are destined to either lose market share to more responsive competitors or (worse) get “disrupted out of existence” by new contenders who lie in wait outside the traditional medical device industry borders — software vendors, systems integrators, and even consumer technology companies.
This was the stern warning issued by Shahid Shah, aka “The Healthcare IT Guy” (and CEO of software consultancy Netspective Communications), during his keynote address at the MD&M West event in Anaheim last month. His talk, Architecting, designing and building medical devices in an outcomes-focused Big Data world, discussed the major market and regulatory forces that have caused healthcare providers to change their purchasing habits. More importantly — for his device manufacturer audience and you — he also identified six key technology trends that must influence your current and future design efforts, if you want to remain relevant in an increasingly connected and data-hungry healthcare space.
1. Commoditization: How much of what’s special in your device has or will become a commodity?
Using inexpensive, readily available, and largely unregulated components such as PCs, tablets, smartphones, and software, providers can now assemble highly capable solutions that are easing their transitions from volume-driven to value-driven healthcare. To an extent, medical device makers should cede this ground to the commodity products, and instead concentrate on integrating the unique capabilities of their medical devices with these off-the-shelf technologies.
2. Consumerization: Can your device be replaced by a mobile phone or other consumer device?
In the “new world order” of healthcare, as Shah characterized it, “smart buyers” are purchasing software-centric medical devices that offer more connectivity and virtualization — much like today’s consumer electronics. He noted that more consumer electronics companies are testing the water in the medical device space, referencing Apple’s recent meeting with the FDA and the rumors surrounding the company’s iWatch (which may or may not do everything from monitor your blood pressure to predict a heart attack). Shah said that the sensors in smartphones could eventually replace many current diagnostic devices, and he went so far as to predict that the mHealth (mobile health) phenomenon could make obsolete “fully half the devices downstairs [at the MD&M West exhibition] in the next 10 years.” Device makers would be wise to ensure their devices have a place in this ecosystem, for instance by designing them to facilitate diagnostic-quality mHealth.
3. Workflow Automation: Can your device fit into agile clinical workflows?
Throw what you think you know about clinical workflows out the window. In the outcomes-focused world of accountable care organizations (ACOs) that we’re moving steadily towards, clinical workflows will change faster than they did under the former fee-for-service model. The next generation of medical devices must be flexible enough to be successfully incorporated into a constantly changing system involving many caregivers (doctors, call centers, family caregivers, etc.) spread across multiple locations (doctor’s offices, hospitals, homes, etc.).
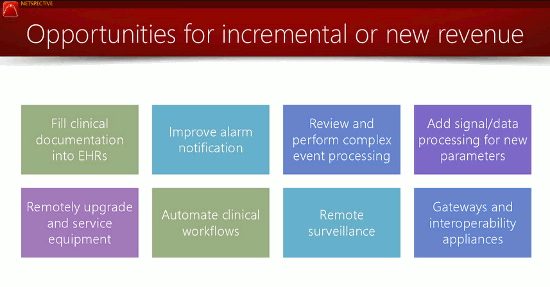
4. Device Interoperability: Can your device connect into the existing IT ecosystem?
Your customers are struggling, Shah said, with interoperability and filling their EHRs (which we’ll get to in a moment) — anything your device can do to simplify their lives will translate to more sales and higher margins. At the very least, your device must be capable of functioning within an IT framework consisting of hundreds or thousands of devices and leveraging dozens of communication protocols (wireless and wired) and data formats. Ideally, your device would enable your customers to establish a “programmable world where devices can coordinate and cooperate with their data collection efforts,” Shah added.
5. Connected EHRs: Can your device fill electronic health records?
Thanks to the Affordable Care Act, providers are under intense pressure to transition to electronic recordkeeping and are investing billions in related software. Unfortunately, few medical devices ease that pressure by pushing data directly to EHR systems. As an example, Shah referenced diagnostic equipment, much of which provides data via an LED screen, requiring the user to read the information and then manually enter it into an EHR system. Few healthcare technology buyers are purchasing standalone, monolithic devices like these when more connected options — particularly those that offer poly-connectivity — exist, according to Shah. Make sure your design expedites EHR data transfer.
6. Accountable Tech: Can your device pay for itself based on diagnostic, therapeutic, or other outcomes?
Being able to prove efficacy of treatment is critical in today’s outcomes-based healthcare economy. Fail to do so, and providers might not get paid — and they expect their technology suppliers to help them bear that burden. Shah said that devices can differentiate themselves by delivering data that helps customers answer questions like: Why is the cost per patient per procedure/treatment going up? Why is the cost of the same procedure/treatment highly variable across localities? What is the effectiveness of a treatment across patients?
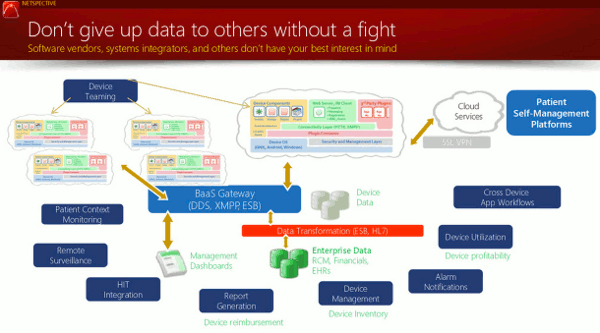
“Hardware, sensors, and software are transient businesses, but data lives forever,” Shah said. “He who owns, integrates, and uses data wins in the end.”
He concluded by pointing out that device makers have a key competitive advantage in the fight over healthcare data: their familiarity with the regulatory battlefield. New and future entrants to the space don’t yet know how to migrate from the unregulated and Class I arena to Class II and III, but they will figure it out eventually. Medical device makers can defend their turf by building effective “data bridges” from their devices to unregulated IT support infrastructure. “Data from devices is too important and specialized to be left to software vendors, managed service providers, and system integrators,” Shah explained. “Don’t give it up without a fight.”
Slides reprinted courtesy of Shahid Shah (www.healthcareguy.com)
