The ABCs Of PCCPs (Predetermined Change Control Plans)
By Randy Horton, Orthogonal

Despite the fact that medical device software can evolve far faster than traditional medical hardware, manufacturers must go through the same resubmission process for a new software version as they would for a new hardware iteration. This regulatory burden facing medical device software and software as a medical device (SaMD) manufacturers is further exacerbated by the speeds at which artificial intelligence (AI) and machine learning (ML) algorithms can learn and improve. Given their potential to improve patient outcomes faster, the industry has called for a more agile regulatory framework that allows AI/ML algorithms to be updated with less regulatory overhead and without compromising safety and effectiveness.
Enter the FDA’s Predetermined Change Control Plans (PCCPs). This regulatory tool aims to ease the regulatory burden of updating AI/ML algorithms and has potential applications across software-enabled medical devices. Companies that master this tool will be able to more rapidly improve their device products – and potentially patient outcomes – faster than their competitors.
In this article, we’ll break down the ABCs of PCCP to help professionals across medtech understand this tool and its nascent potential:
- For R&D team members, PCCP can help them push post-release updates faster and iterate more frequently without lengthy FDA resubmissions.
- For regulatory and quality personnel, PCCP can help simplify compliance and reduce their overall regulatory strategy and compliance burden.
- For attorneys in the medtech space, PCCP can help minimize legal risks and provide a clear legal and regulatory pathway that mitigates compliance issues.
- For project managers, PCCP can help them manage more predictable timelines and achieve a faster time to market.
- For device executives, PCCP can help them gain a competitive advantage by accelerating innovation and improving patient outcomes.
What Is PCCP?
At a high level, PCCP is a regulatory tool that allows manufacturers to regularly update AI/ML without having to go to the FDA for approval. Manufacturers initiating a PCCP present regulators with documentation outlining the scope of changes and methods of making them. Regulators review the documentation and establish an agreement on what changes can be implemented within strict guidelines without refiling. This ensures safety and effectiveness while allowing companies to improve algorithms faster.
To use an analogy, a PCCP is similar to a parent setting a curfew for their teenager. The teen is allowed a measure of freedom – they can hang out with their friends and wander around the neighborhood – but they must stay within a certain range, not go to any parties with alcohol, and be back home by 11 p.m. sharp, or face the consequences.
While AI/ML is the FDA’s focus for PCCP, the legal language around it does not limit it to AI/ML. Other forms of medical device software (and possibly even hardware) could potentially take advantage of PCCP. Orthogonal and MedSec’s white paper “Making the Significant Insignificant: Implementing a PCCP for Class II SaMD Products Beyond AI/ML” examines the full legal text of PCCP and proposes key components for Class II SaMD products.
For an example of the power of PCCP, take a look at the following table of approved changes from the 2023 resubmission filing of Apple’s Irregular Rhythm Notification Feature for Apple Watch. Apple can refine its AI algorithm’s ability to detect atrial fibrillation without going through the lengthy resubmission process, resulting in faster, more accurate alerts for users.
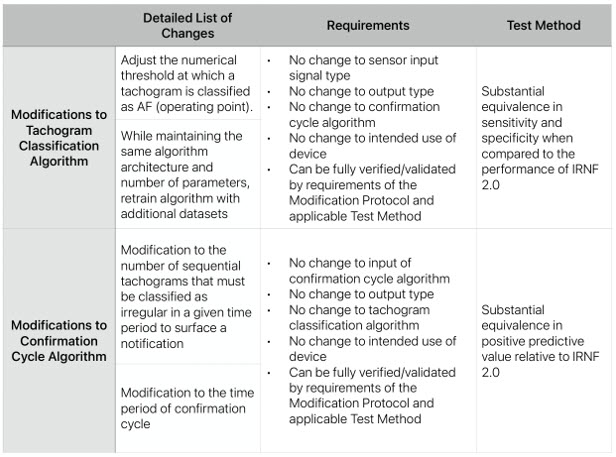
Apple’s K231173, an example of an FDA-cleared PCCP. Source: FDA
PCCP shares similarities with a previous FDA initiative, the Pre-Cert Program. In that pilot program, the FDA reviewed medical device companies’ quality processes before development, allowing approved companies a fast track to bring future devices to market with less regulatory oversight. (The Pre-Cert program was discontinued in 2022 due to concerns that the FDA had overstepped its Congressionally approved boundaries.) In contrast, legal language specifically authorizing PCCP was passed in the 2023 Congressional Omnibus Bill, indicating Congressional support for the broader principles behind both programs.
What Kinds Of Medical Devices Benefit From PCCP?
Medical devices with AI/ML algorithms are the major beneficiaries of PCCP. Under a PCCP, AI/ML algorithms can be updated by manufacturers as frequently as every week, compared to refiling, which can take several months through the standard regulatory submission process. Allowing for more frequent updates of AI/ML should lead to even more effective algorithms with a greater ability to detect disease and injury.
What Goes Into A PCCP?
A PCCP has three key components:
- A list of planned future modifications
- The change control process, or predetermined protocols for developing, verifying, validating, and implementing those modifications
- A risk management process and testing strategy for assessing the impact of any modifications
Based on the experiences of companies that have successfully submitted PCCPs, the FDA expects a significantly higher level of detail for a PCCP than a typical pre-market submission. If a PCCP is less than 10 pages long, it’s probably not detailed enough.
What Are The Limits Of PCCP?
PCCP gives manufacturers the latitude to make changes to their device algorithms within pre-approved limits and without going through the lengthy resubmission process (instead, manufacturers submit a Letter to File). Changes made to the device must fall within its intended use, indications for use, and risk profile. For example, a PCCP as currently implemented by the FDA will not allow a change in a device’s indication from 18 years of age through 82 years of age to treating patients from 18 to 95, even if the company has data proving its effectiveness for this older age group.
While PCCP may alter the submission process for device updates, it’s not a pass to ignore existing quality processes. Manufacturers must continue to follow their quality management systems and verify the data used to test algorithms meets the agreed-upon performance standards.
What Other Challenges Do Manufacturers Face When Submitting A PCCP?
As the FDA’s PCCP draft guidance was released in April of 2023 and is still accepting comments as of this article’s publication, both manufacturers and regulators need time to acclimate to it. Regulators may be cautious in allowing certain changes to be made without their oversight, particularly in submission types where no other PCCP has been authorized.
Nonetheless, it’s the hope of Orthogonal and other industry leaders that as regulators gain more confidence in reviewing and approving PCCPs, they will be more comfortable being accommodating and flexible with manufacturers. We have observed a similar pattern with the FDA and SaMD. Early on, regulators required manufacturers to prove that patients knew how to open an app, whereas now they do not need such reassurance. As more PCCPs are approved covering different kinds of algorithms and device functions, future PCCPs should be able to draw on them as precedents, further easing the PCCP approval process.
Conclusion
PCCP is more than just a regulatory tool for updating AI/ML algorithms in medical devices. It represents a crucial step toward regulations that reflect the iterative nature of software products. We hope and expect that PCCP will be embraced by both regulators and manufacturers and that it will serve as an inflection point for change in the FDA’s regulatory submission process for medical device software and SaMD.
About The Author:
 Randy Horton is chief solutions officer at Orthogonal, a software consulting firm. Horton serves as co-chair for AAMI’s Cloud Computing Working Group, as well as AAMI CR:510(2021) and the in-process Technical Information Report #115, all of which address how to safely move medical device computing functions into the cloud. He is a frequent speaker at conferences and webinars, including events hosted by AdvaMed, AAMI, HLTH, RAPS, and the Human Factors and Ergonomics Society (HFES).
Randy Horton is chief solutions officer at Orthogonal, a software consulting firm. Horton serves as co-chair for AAMI’s Cloud Computing Working Group, as well as AAMI CR:510(2021) and the in-process Technical Information Report #115, all of which address how to safely move medical device computing functions into the cloud. He is a frequent speaker at conferences and webinars, including events hosted by AdvaMed, AAMI, HLTH, RAPS, and the Human Factors and Ergonomics Society (HFES).
