Understanding The Impact Of An RMP On Patient Risks Using Relational Risk Analysis
By Mark F. Witcher, Ph.D., biopharma operations subject matter expert

Health Canada recently released a new guideline for managing patient risks of a new or significant drug or therapy that provides an opportunity to better describe and understand a patient’s benefit/harm risks.1 The guidance describes the submission of a risk management plan (RMP) for identifying and managing adverse reactions (ARs). This article describes how relational risk analysis (ReRA) can be used to build a system risk structure (SRS) for understanding the critical role of an RMP, and similar programs including EMA’s RMP and FDA’s REMS, for minimizing the likelihood of significant harm to patients from a new therapy.2-7
An RMP provides proactive methods of identifying and treating ARs in patients. The RMP would be developed when either the developing company or regulatory agencies are concerned about the uncertainty of a therapy causing harm risks to patients.
The RMP has two critical functions for increasing patient safety. The first is identifying what patient responses to look for with respect to detecting ARs to quickly initiate appropriate treatment. The second is to identify and describe appropriate treatment options for the ARs. Per the guidelines, both the ARs and treatment options must be prospectively identified and described to the extent possible by the therapy’s development team based on their knowledge of the therapy, clinical trial experience, and from numerous sources of information, knowledge, and experience, many described in the regulatory guidance.
Understanding the impact of pharmaceuticals and medical device risks on patient health is critical to the therapy’s development and continued use. ReRA can provide considerable insights into how these risks can be structured and analyzed to maximize patient safety.
Before structuring the RMP into a patient risk analysis, let’s review a summary of ReRA.
Relational Risk Analysis (ReRA)
ReRA defines and models risks as “the impact of uncertainty on a mechanism, process, or system that produces an objective or consequence.” A useful working ReRA definition is “a risk is the failure of a risk mechanism to achieve its objective.”
ReRA views risk as relationships between events with a cause or initiating event passing through a connecting mechanism that has a likelihood, expressed as a single trial Bernoulli probability, of propagating the cause event to produce an objective or consequence. The risk relationship for a risk mechanism achieving either a harm or benefit event is summarized in Figure 1.
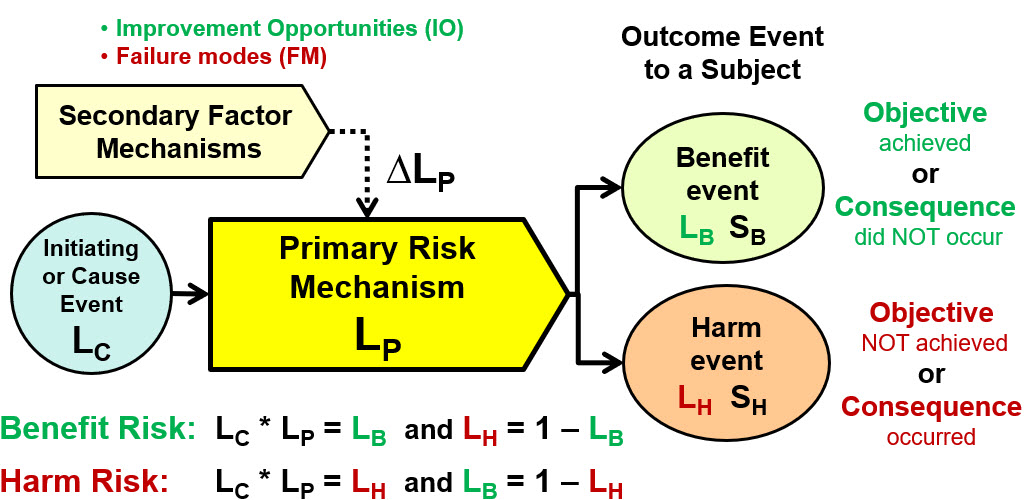
Figure 1: This shows a ReRA SRS element for describing how a risk either achieves a beneficial objective or prevents a harmful consequence. All likelihoods are expressed as probabilities. The initiating cause event of likelihood LC passes through the risk’s primary mechanism to result in either a benefit event of probability LB and significance of SB or a harm consequence event of probability LH and severity SH. For a benefit risk, the objective of the primary mechanism is to maximize the likelihood LB of achieving the benefit thus minimizing the likelihood of a harm consequence. For a harm risk, the objective of the primary mechanism is to minimize the likelihood of the harm consequence thus maximizing the likelihood of the benefit event.
The SRS element has the following components:
- Initiating or cause event – a causal event of likelihood LC that enters the primary mechanism. For many risks, particularly administering a therapy, the cause event is deliberate with a LC = 1.
- Primary risk mechanism – any combination of equipment, people, actions, or anything else that takes the initiating event and produces an objective event with a likelihood of LP. For a benefit risk, the likelihood of a benefit is LB = LC * LP and LH = 1 – LB. For a harm risk, the likelihood of the harm is LH = LC * LP and LB = 1 – LH.
- Benefit event – the beneficial event to the subject with a likelihood of LB and a significance of SB. The benefit is achieving an objective or preventing a harmful consequence.
- Harm event – the harm event to the subject with a likelihood of LH and a severity of SH. The harm event is not achieving a beneficial objective or the occurrence of a harmful consequence.
- Secondary factor mechanism – a secondary risk or risk event that can change the performance LP of the primary risk mechanism by ∆LP for either increasing the likelihood of it achieving its objective as an improvement opportunity (IO) or increasing the likelihood of a harm consequence as a failure mode (FM).
For analyzing a complex multi-mechanism risk, the basic SRS element in Figure 1 can be used to form a continuous sequence of primary mechanisms required to link an initiating event to an objective or consequence event impacting a subject. The risk must always be connected to the final subject of the risk, in this case the patient. If any of the initiating cause event, primary risk mechanism, outcome event, or the risk’s subject changes, a different risk is described.
The objective form shown in Figure 1 describes two different types of risks. The first is a “harm risk” where the objective of the primary mechanism is to minimize the propagation of the initiating event, making the likelihood of the harm event occurring acceptably small. The harm risk model is frequently used for safety and simple contamination risks.8,9 The second type is a “benefit risk” where the objective of the primary mechanism is to maximize the likelihood of producing the benefit event. Benefit risks can be used to model a wide variety of risks that include supply chains, procedures, and high-value contamination risks.9,10,11
Because ReRA models both types of risks, the method requires an efficient approach for modeling and communicating both very low and very high probabilities. The rating system used is described in Appendix A. While likelihood can be used directly, the rating scale provides an efficient approach for describing and managing likelihoods. The notation for the likelihood LX is LX^. The likelihood rating system is explained in detail along with its use for describing harm and benefit risks in the references.8,9
Structuring Patient Therapy Risks
ReRA is particularly valuable for modeling patient outcomes of either a benefit or harm. The SRS in Figure 2 describes the flow of events from using the therapy on the patient (event 1) to either a benefit event (2a) or serious harm event (4b).
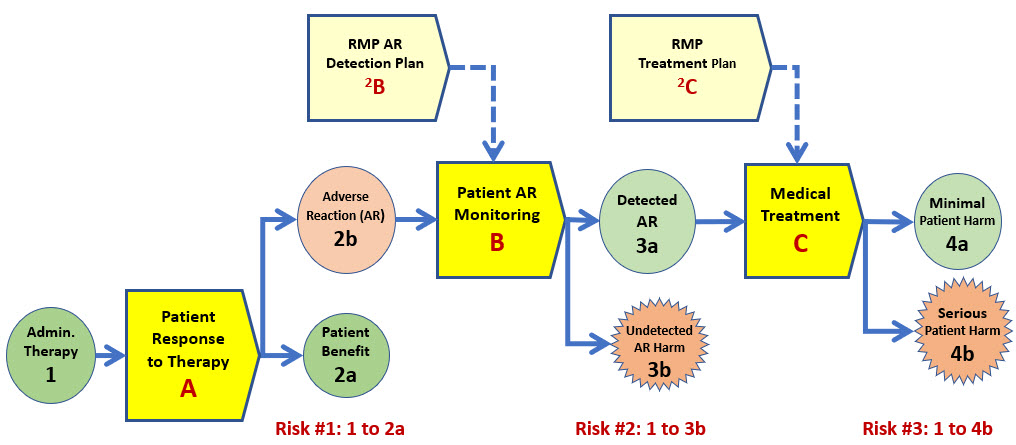
Figure 2: This depicts one of several possible SRSs for the risk to a patient of administering a therapy possibly resulting in either a benefit or serious harm. In this SRS scenario, system A is the response of the patient to receiving the therapy. System B is monitoring the patient to detect an AR, and system C is the medical treatment the patient receives if an AR is detected. By convention, risk mechanisms are labeled by letters and events by numbers.
The SRS shown in Figure 2 has the following three primary risk mechanisms that separate giving the therapy to the patient and the patient experiencing serious harm:
- System A – The patient’s response to receiving the therapy. The therapy produces either a benefit (2a) or an AR (2b).
- System B – Monitoring of the patient’s response to the therapy for detecting an adverse reaction (AR). The outcome of the monitoring mechanism is either detecting an AR (3a) so treatment can begin or not detecting an AR (3b). For example, a possible undetected AR might be cancer. An undetected AR is viewed as a harm event because the risk model is assuming the reason an AR is absent is because the therapy is effective. The risk mechanisms for eventually identifying and treating or dealing with an undetected AR are not included in the analysis.
- System C – The medical treatment provided to treat an AR produced by the therapy. The benefit of system C is successfully treating the patient (4a). If the patient treatment is not successful, the serious patient harm event (4b) would be the risk’s consequence.
ReRA defines a risk as a sequence of events and their connecting mechanism from an initial event to a final event that impacts the subject.
Analyzing Patient Risks
The SRS shown in Figure 2 can be used to analyze three different patient risks.
- Risk #1 – The benefit to the patient of being given the therapy (event 1 to 2a).
- Risk #2 – Harm to the patient from an undetected AR (1 to 3b).
- Risk #3 – Harm to the patient given the therapy from a treated AR (1 to 4b).
The three risks will be used to demonstrate how ReRA can structure the risk relationships for analysis. The primary goal of analyzing each risk is to estimate the likelihood of occurrence for the three outcomes. The analysis of the second two risks will include the impact of the RMP on systems B and C by including the RMP’s secondary risk mechanisms in the SRS.
For those not familiar with ReRA’s risk likelihood rating method, a summary is provided in Appendix A. For this article, the likelihood ratings are for illustrating ReRA. The actual estimated likelihoods for a specific analysis can vary widely based on the specific therapy and the patient populations being treated.
The likelihoods used in this article are for demonstration purposes only. Likelihood values for a specific therapy mechanism for patient population must be estimated by qualified teams of medical and regulatory personnel. In addition, the discussion in the example risks have been kept to an absolute minimum to keep the length of the article as concise as possible.
Severity and likelihood ratings must be estimated by a qualified team of medical and therapy experts using all the knowledge and experience available for building the RMP. The team will be challenged to reach a consensus estimate for the probabilities. However, the likelihood rating system only requires order-of-magnitude estimates for initial acceptance decision-making. The true value of the analysis is not in the numbers but in the discussion and acceptance decisions for identifying and managing the medical threats to the patient.
Initially reading and understanding ReRA risk registers (RR) takes some effort. But after studying both the SRS and RR together, they can be interpreted very quickly to facilitate discussions among the analysis team for reaching a consensus.
The severity of the various risk events on the patients is a medical issue dependent on the treatment, the patient population, and the possible ARs. Severities are likely significant and obvious and thus are beyond the scope of this article.
Risk #1 – Patient benefiting from therapy
Table 1 shows the RR for the risk of the therapy benefiting the patient. The first risk is analyzed as a benefit risk. If the benefit does not occur, then an adverse reaction (AR) is experienced by the patient.
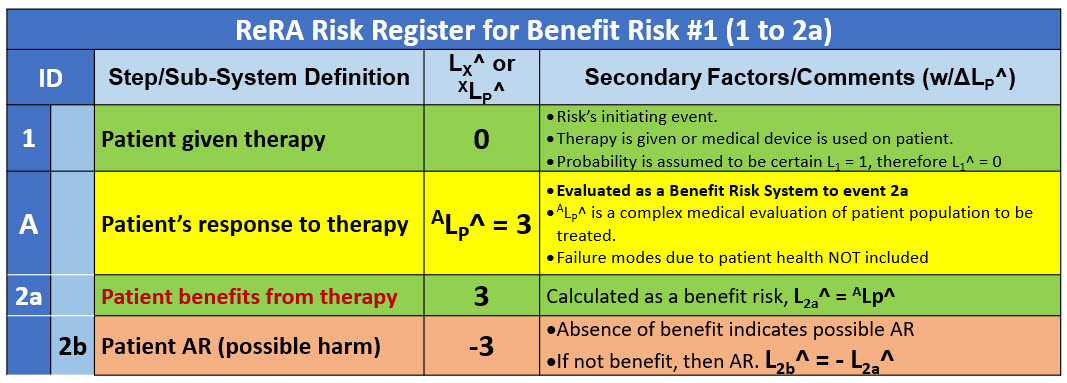
Table 1: Summary RR for Risk #1 of the therapy benefiting the patient (event 2a). A parallel risk is the patient having an AR to the therapy (2b).
The medical team, based on their experience and available information, would estimate and communicate the expected effectiveness of the therapy using the likelihood rating system in Appendix A. The likelihood of the benefit is determined using the relationship ALP^ = L2a^. As described in Appendix A, the likelihood of the AR (2b) is thus L2b^ = - L2a^.
A full evaluation of ALP^ might also include a variety of improvement opportunities (IO) and failure modes (FM) associated with the patient’s medical history and condition of the patient population being given the therapy.
Risk #2 – Patient harm from undetected AR
The second risk (1 to 3b) is described in the risk register in Table 2. The initiating event (1) passes through system A and system B to reach the event of patient harm due to an undetected AR because of the patient monitoring program not detecting the AR, leaving the harmful medical condition untreated (3b).
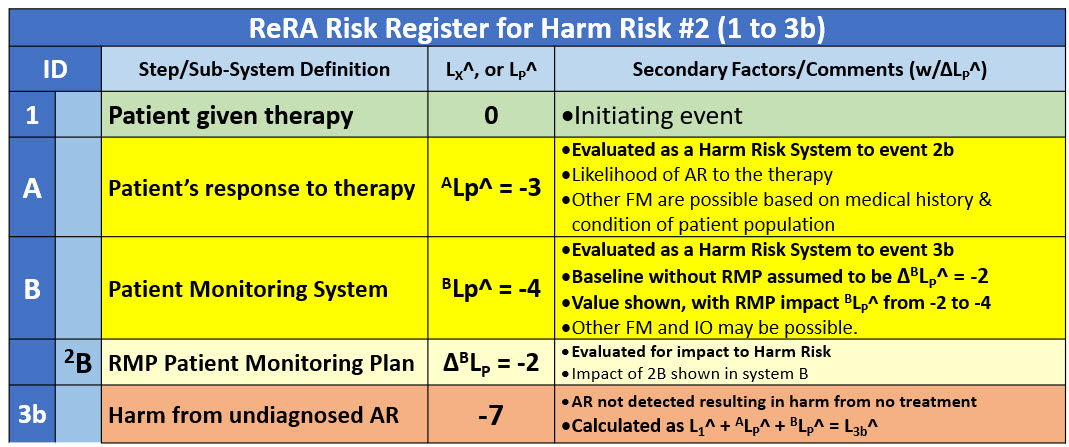
Table 2: Risk register for risk #2 of an undetected AR being untreated, resulting in harm to the patient (3b). The RMP’s impact on system 2B is described by system 2B. In this example, the RMP is estimated to result in a two-order-of-magnitude improvement in the performance of system B.
The impact of system A remains the same as risk #1 except the likelihood rating is switched from a benefit risk value of 3 to a harm risk performance rating value of -3. The baseline performance of system B, evaluated as a harm risk system, is assumed to have a rating of -2. However, the RMP is estimated by the analysis team to have a significant IO impact on system B. Under the assumptions described in the RR, the RMP is assumed to improve the performance of system B by two orders of magnitude, giving system B a performance rating of - 4. As shown in Table 2the two systems provide the patient a likelihood rating of L3b^ = -7 of the therapy harming the patient by an undetected and untreated AR.
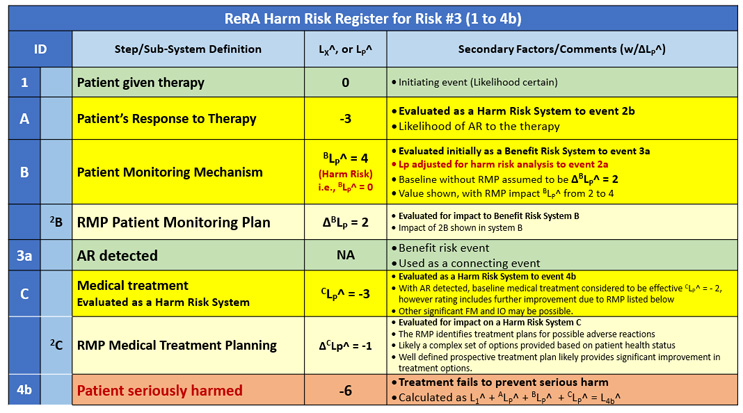
Risk #3 – Patient harm after treatment
The final risk is the patient being harmed by the therapy if an AR is detected and treated. The RR for the third risk is shown in Table 3. The risk of significant harm event 4b occurs by passing through all three systems as shown in Figure 2.
Table 3: Risk register for risk #3 of the therapy seriously harming the patient. The risk’s consequence (4b) results from the medical treatment not successfully treating the AR, resulting in the patient being seriously harmed.
For preventing the serious patient harm (4b), a three-system sequence protects the patient from harm. System A describes how likely the patient population is to experience an AR. In essence, the patient is “protecting” themself from harm by successfully tolerating the therapy. System A is an extremely complex patient system because it describes the internal health mechanisms of a large population of people that determines their typical or expected response to the therapy. The many potential FMs and IOs of system A are not included in this analysis but would likely be a very important part of a detailed risk analysis for a specific therapy/patient risk.
System B only protects the patient from risk #2 but does not “protect” the patient from the final harm 4b. From a risk modeling standpoint, system B only provides an effective connection to system C through a beneficial event (3a). Obviously, the more effective system B is at detecting the AR, the more it protects the patient from the harm of risk #2.
System C protects the patient from serious harm by treating the AR to minimize its impact on the patient. System C is again a very complex set of subsystems with many FMs and IOs that can be considered in the analysis. This analysis in Table 3 limits the IOs to just the RMP provided to the medical personnel treating the patients as described by secondary system 2B. In this example, the RMP improves the performance of system C by one order of magnitude. For complex therapies, the improvement potential for the RMP (secondary system 2B) could be much higher.
Discussion/Conclusions
This article describes a risk analysis approach a team of medical experts can use to identify and analyze the impact of secondary risk factors on primary risk mechanisms for managing patient safety risks. Specifically, the approach provides a method of understanding the potential impact of a therapy RMP on the benefit and harm risks associated with giving patients a new or highly impactful therapy. The risk analysis approach also can assist with the initial regulatory and product development analysis necessary for deciding to require an RMP. While this article does not describe a specific RMP, it does demonstrate a risk analysis method that can facilitate the development and understanding of RMPs and how to better develop and use them for improving patient safety.
The use of ReRA/SRS also can support and aid with the possibilities of significant disagreements within a team of medical experts. The simple rating scale forces teams to reach a consensus on the likelihood rating estimates necessary for making medical decisions, detecting ARs, and treatment decisions for effective RMPs. The straightforward likelihood rating system allows little room for false subjective alignments common with risk matrices using subjective rating scales.
Depending on the severity of patient harm at events 2b, 3b, or 4b for specific therapies and patient populations, a very comprehensive risk analysis, including identifying and analyzing the impact and likelihoods of all the significant secondary factors for all three primary systems would be appropriate. The analysis also can be expanded by dividing the three primary systems into a variety of subsystems to better describe the flow of events to important patient outcomes.
Future Work – Patient Risk/Benefit Risk Assessments
ReRA is a unique risk analysis approach because it explicitly identifies both a risk’s mechanisms and events. Because ReRA identifies the mechanisms by which the events are connected, the mechanisms, systems, and processes become available for analysis, evaluation, and management to control the likelihood of both beneficial objectives and harm consequences occurring to patients.
While the current version of ReRA uses single-trial Bernoulli probability distributions, further development of future ReRA methods could support the use of more sophisticated and perhaps more appropriate probability distributions. These distributions could aid in better describing both input cause and failure mode events as well as facilitate the use of sophisticated system probability distributions for describing how the mechanisms propagate the input events into output events. Improved probability distributions could be used to support a Monti Carlo analysis of the output benefit objectives and harm consequences.
ReRA remains in its early development phase with the basic ReRA element shown in Figure 1 and, as demonstrated in Figure 2, provides a robust foundation for analyzing complex risks and multi risk landscapes.
References
- Submitting risk management plans guidance document, Health Canada, February 24, 2025
- REMS Logic Model: A Framework to Link Program Design with Assessment DRAFT Document, FDA, May 2024.
- Format and Content of a REMS Document, FDA, January 2023
- Guidance on the format of the risk management plan (RMP) in the EU – in integrated format, EMA, October 31, 2018.
- Good Pharmacovigilance Practices (GVP) Guidelines – GUI-0102, Health Canada, February 11, 2013.
- Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment, FDA, March 2005
- ICH E2E Pharmacovigilance Planning, ICH Harmonized Tripartite Guideline, November 2004
- Witcher, M.F., A New Approach for Minimizing Human Errors in Biopharmaceuticals and Medical Devices, Bioprocess Online, February 3, 2025. https://www.bioprocessonline.com/doc/a-new-approach-for-minimizing-human-errors-in-biopharmaceuticals-and-medical-devices-0001
- Witcher, M.F., Managing Contamination Risks in the Pharmaceutical and Medical Device Industries Using Relational Risk Analysis, BioProcess Online, February 2, 2025. https://www.bioprocessonline.com/doc/managing-contamination-risks-in-the-pharmaceutical-and-medical-device-industries-using-relational-risk-analysis-0001
- Witcher, M.F., Using Relational Risk Analysis to Control Procedure Failures, February 15, 2024. https://www.bioprocessonline.com/doc/using-relational-risk-analysis-to-control-procedure-failures-in-the-bio-pharma-medical-device-industry-0001
- Witcher, M.F., Managing Supply Chain Risks Using Relational Risk Analysis, April 5, 2024. https://www.meddeviceonline.com/doc/managing-supply-chain-risks-using-relational-risk-analysis-0001
Appendix A: Communicating and Managing a Risk’s Probabilities
All the probabilities currently used in ReRA are assumed to be single trial Bernoulli distributions, meaning that the event occurs or does not occur with the stated probability. Quickly and efficiently describing a risk’s probabilities over the entire range from essentially certain (100%) to essentially impossible (0%) is critical to any risk analysis. Table A1 provides a method of communicating probabilities according to the rating scale so an analysis team can reach a consensus on probability values.
The probability rating scale from ≥ 7 “essentially certain” to ≤ -7 “essentially impossible” shown on the right of the table can be used to communicate the probabilities shown in the left.
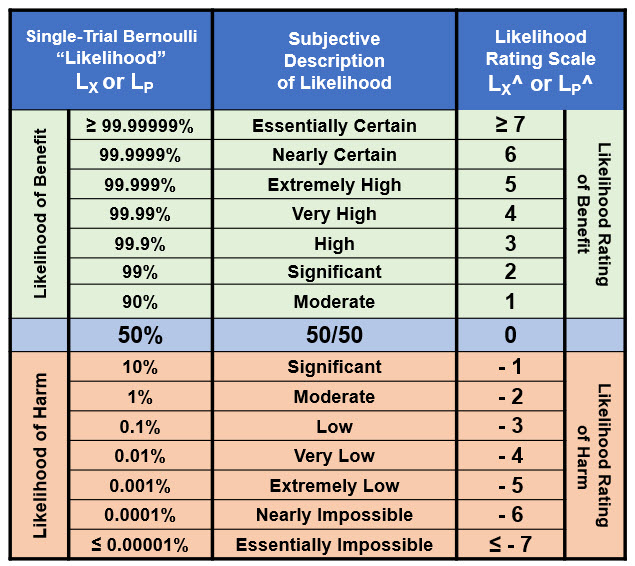
Table A1: The range of probabilities from essentially certain to essentially impossible for an event X’s probability of occurrence (LX) and a system’s probability of propagation SysLP. The ranges can be extended in either direction if needed. Since there is no certainty, the ratings for LX = 0 or 1 are undefined.
The LX^ rating scale is symmetric around 50% with positive ratings for probabilities > 50% and negative values for probabilities < 50%. The probabilistic relationship LX + ¬LX = 1 is equivalent to LX^ + ¬LX^ = 0, resulting in ¬LX^ = – LX^ for translating likelihood ratings between failures and success and vice versa.
When using the table for harm risks, for all LX ≥ 0.5, LX^ = 0 and for benefit risks, all LX ≤ 0.5, LX^ = 0. For harm risks with LX values less than 50%, adding the ratings values is equivalent to multiplying the probabilities.
About The Author:
 Mark F. Witcher, Ph.D., has over 35 years of experience in biopharmaceuticals. He currently consults with a few select companies. Previously, he worked for several engineering companies on feasibility and conceptual design studies for advanced biopharmaceutical manufacturing facilities. Witcher was an independent consultant in the biopharmaceutical industry for 15 years on operational issues related to: product and process development, strategic business development, clinical and commercial manufacturing, tech transfer, and facility design. He also taught courses on process validation for ISPE. He was previously the SVP of manufacturing operations for Covance Biotechnology Services, where he was responsible for the design, construction, start-up, and operation of their $50-million contract manufacturing facility. Prior to joining Covance, Witcher was VP of manufacturing at Amgen. You can reach him at witchermf@aol.com or on LinkedIn (linkedin.com/in/mark-witcher).
Mark F. Witcher, Ph.D., has over 35 years of experience in biopharmaceuticals. He currently consults with a few select companies. Previously, he worked for several engineering companies on feasibility and conceptual design studies for advanced biopharmaceutical manufacturing facilities. Witcher was an independent consultant in the biopharmaceutical industry for 15 years on operational issues related to: product and process development, strategic business development, clinical and commercial manufacturing, tech transfer, and facility design. He also taught courses on process validation for ISPE. He was previously the SVP of manufacturing operations for Covance Biotechnology Services, where he was responsible for the design, construction, start-up, and operation of their $50-million contract manufacturing facility. Prior to joining Covance, Witcher was VP of manufacturing at Amgen. You can reach him at witchermf@aol.com or on LinkedIn (linkedin.com/in/mark-witcher).