What You Should Know About The FDA's New Final Rule On LDTs
By Mahnu V. Davar, Philip R. Desjardins, Abeba Habtemariam, and Phillip V. DeFedele, Arnold & Porter

On May 6, 2024, the U.S. Food and Drug Administration (FDA or the agency) published its highly anticipated final rule, which was formally published in the Federal Register on May 6, revising the regulatory definition of an in vitro diagnostic (IVD) product to explicitly capture IVDs manufactured by laboratories.
This follows the absence of proposed congressional action and FDA’s review and consideration of comments to the October 2023 proposed rule (described in our prior Advisory on this subject) resulting in modifications to its phaseout policy and continued exercise of enforcement discretion for certain tests. In connection with the final rule, FDA also issued two draft enforcement policies for certain tests offered in response to emergent situations or public health emergencies (PHEs). Despite the potential for legal challenges to the final rule, clinical laboratories should begin thinking about strategies for evaluating whether their laboratory developed tests (LDTs) are subject to FDA’s phaseout policy, determining the extent of FDA requirements that apply to such LDTs, and engaging with FDA.
What Did the Final Rule Do?
The final rule amended the definition of an IVD in 21 C.F.R. § 809.3 to make clear that these products are devices as defined under the Federal Food, Drug and Cosmetic Act (FDCA), and may also be biological products under the U.S. Public Health Service Act, “including when the manufacturer of these products is a laboratory.” Although not a substantial re-write of the IVD definition, this added phrase makes FDA’s position clear that LDTs are subject to regulation as, at a minimum, devices.
Why Does This Matter?
Historically, FDA exercised enforcement discretion for LDTs, declining to impose its device authorities over such tests in most instances. For purposes of this enforcement discretion policy, the agency defined LDTs as IVDs intended for clinical use that were designed, manufactured, and used within a single clinical laboratory certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) that meets CLIA regulatory requirements to perform high complexity testing. As such, LDT manufacturers that generally operated outside FDA oversight will now be expected to come into compliance with FDA requirements and controls applicable to their tests. In consideration of this substantial operational and compliance burden, the preamble to the final rule details a phaseout policy under which FDA will gradually end its general LDT enforcement discretion policy in five phases over a four-year period.
What Tests Are Subject to the Phaseout Policy?
The phaseout policy generally applies to LDTs as defined above, as well as “IVDs offered as LDTs,” meaning tests that are manufactured and offered as LDTs by CLIA-certified laboratories that meet CLIA requirements to perform high complexity testing and that are used within such laboratories, even if they do not fall within FDA’s historical understanding and definition of an LDT because they are not designed, manufactured, and used within a single laboratory. Thus, the policy technically applies to a broader range of tests than those that actually meet FDA’s definition of an LDT. However, despite this breadth, the final rule makes clear that the phaseout policy does not extend to IVDs manufactured or used outside of a laboratory, including collection devices.
Consistent with the proposed rule, FDA made clear that certain tests would be excluded from the phaseout policy and subject to immediate regulation since they were never eligible for enforcement discretion:
- Direct-to-consumer tests
- Tests intended as blood donor screening or human cells, tissues, and cellular and tissue-based products donor screening tests required for infectious disease testing under 21 C.F.R. § 610.40 and 21 C.F.R. § 1271.80(c), respectively, or required for determination of blood group and Rh factors under 21 C.F.R. § 640.5
- Tests intended for actual or potential emergencies or material threats declared under Section 564 of the FDCA
Further, the final rule confirmed that FDA would continue exercising enforcement discretion (and thus not apply device requirements) to the following tests originally described in the proposed rule:
- “1976-Type LDTs” (i.e., tests that use manual techniques without automation performed by laboratory personnel with specialized expertise, use components legally marketed for clinical use, and are designed, manufactured, and used within a single CLIA-certified laboratory meeting CLIA requirements for high complexity testing)
- Human Leukocyte Antigen (HLA) tests that are designed, manufactured, and used within a single CLIA-certified laboratory meeting CLIA requirements for high complexity histocompatibility testing and used for HLA allele typing in connection with organ, stem cell, and tissue transplantation, HLA antibody screening and monitoring, or conducting real and “virtual” HLA crossmatch tests
- Tests intended solely for forensic or law enforcement purposes
- Tests used solely for public health surveillance when intended solely for use on systematically collected samples for analysis and interpretation of health data for disease prevention and control and the test results are not reported to patients or their healthcare providers
Notably, in consideration of comments and other feedback, FDA decided to continue to exercise full or partial enforcement discretion for the following tests:
- LDTs manufactured and performed within the Veterans Health Administration or the Department of Defense (full enforcement discretion continues)
- LDTs approved by the New York State Clinical Laboratory Evaluation Program (enforcement discretion continues for premarket review requirements)
- Non-molecular antisera LDTs for rare red blood cell antigens where such tests are manufactured and performed in blood establishments, including transfusion services and immunohematology laboratories and where there is no alternative available to meet the patient’s need for a compatible blood transfusion (enforcement discretion continues for premarket review requirements and all Quality System (QS) requirements other than the recordkeeping requirements at 21 CFR 820 (Subpart M))
- Currently marketed IVDs offered as LDTs that were first marketed prior to the date of issuance of the Final Rule (May 6, 2024) and that are not modified or are modified in certain limited ways (enforcement discretion continues for premarket review requirements and all QS requirements other than the recordkeeping requirements at 21 CFR 820 (Subpart M))
- LDTs manufactured and performed by a laboratory integrated within a healthcare system to meet an unmet need of patients receiving care within the same healthcare system (enforcement discretion continues for premarket review requirements and all QS requirements other than the recordkeeping requirements at 21 CFR 820 (Subpart M))
For those tests subject to only partial enforcement discretion, the final rule makes clear that all other requirements would apply as they are phased-in under the general phaseout policy for all IVDs offered as LDTs.
What Is The Phaseout Policy?
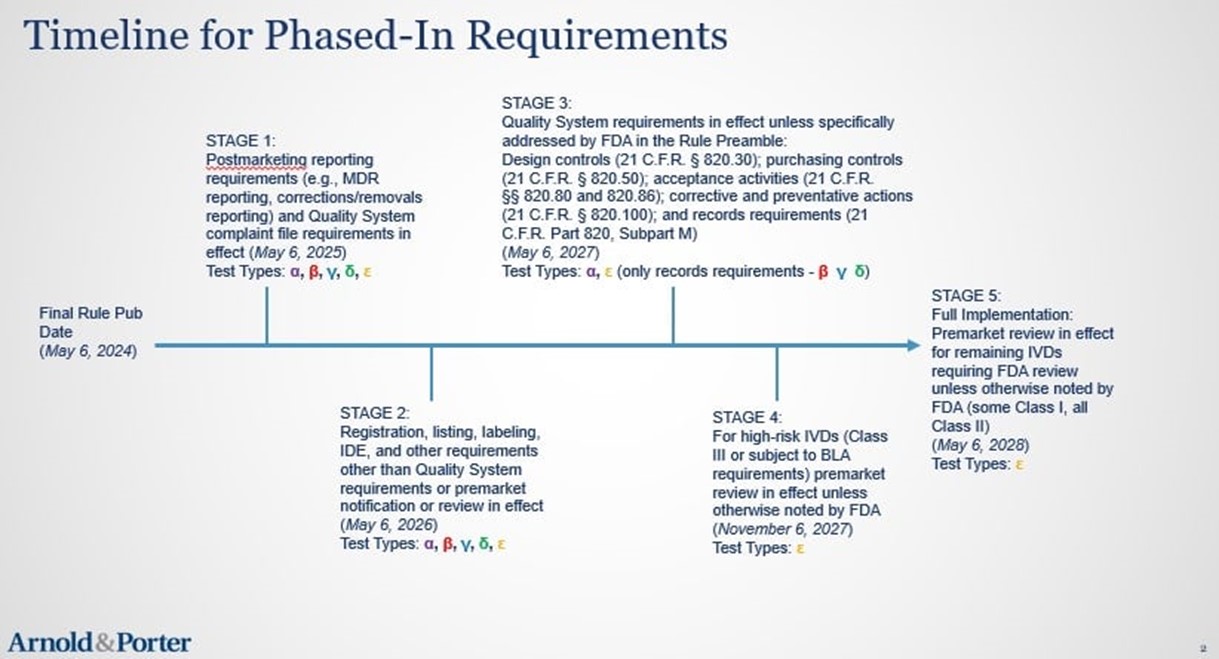
The phaseout policy consists of the following five stages, which start on May 6, 2024:
Stage 1: FDA will end the general enforcement discretion policy as to medical device safety reporting, correction and removal requirements, and QS complaint file requirements one year after publication of the final rule. Thus, manufacturers of all IVDs offered as LDTs that are not subject to FDA’s continued exercise of full enforcement discretion must come into compliance with these requirements by May 6, 2025.
Stage 2: FDA will end the general enforcement discretion policy as to all other medical device requirements, except for QS and premarket review requirements, two years after publication of the final rule. Therefore, manufacturers of all IVDs offered as LDTs that are not subject to FDA’s continued exercise of full enforcement discretion must come into compliance with these requirements by May 6, 2026.
Stage 3: FDA will end the general enforcement discretion policy as to the following QS requirements three years after publication of the final rule: (1) design controls (21 C.F.R. § 820.30); (2) purchasing controls (21 C.F.R. § 820.50); (3) acceptance activities (21 C.F.R. §§ 820.80 and 820.86); (4) corrective and preventative actions (21 C.F.R. § 820.100); and (5) records requirements (21 C.F.R. Part 820, Subpart M). As such, manufacturers of all IVDs offered as LDTs that are not subject to FDA’s continued exercise of full enforcement discretion or partial enforcement discretion as to certain QS requirements must come into compliance with these requirements by May 6, 2027.
Stage 4: FDA will end the general enforcement discretion policy as to premarket review requirements for high-risk IVDs (i.e., Class III IVDs or IVDs subject to Biologics License Application requirements) three and a half years after publication of the final rule. Manufacturers of all IVDs offered as LDTs that are not subject to FDA’s continued exercise of full enforcement discretion or partial enforcement discretion as to premarket requirements must come into compliance with these requirements by November 6, 2027. Notably, on its media call regarding the final rule, FDA stated that it intends to complete the reclassification of certain Class III IVDs to Class II IVDs in advance of this deadline, thus lessening the number of PMAs that will be submitted. We further discuss this initiative and its potential relation to the final rule in our related Advisory on this topic.
Stage 5: FDA will end the general enforcement discretion policy as to premarket review requirements for all remaining IVDs requiring FDA review unless otherwise noted by FDA (i.e., some Class I and all Class II IVDs) four years after publication of the final rule. Manufacturers of all IVDs offered as LDTs that are not subject to FDA’s continued exercise of full enforcement discretion or partial enforcement discretion as to premarket requirements must come into compliance with these requirements by May 6, 2028.
Overview Of Test Types And Status
| Subject to immediate regulation | Partial enforcement discretion | Complete enforcement discretion |
|---|---|---|
|
|
|
| General Phase-in Policy: All other IVDs manufactured and offered as LDTs by CLIA-certified laboratories meeting CLIA requirements for high complexity testing and used within such laboratories (“ε”) | ||
Source: Arnold & Porter
Source: Arnold & Porter
What About The Draft Public Health Emergency Guidance?
Although the draft PHE policies both address certain tests offered in response to emergent situations or public health emergencies, one relates to tests offered prior to issuance of a declaration under Section 564 of the FDCA while the other relates to tests offered during a declared emergency. These are notable because FDA explains in the final rule that its general enforcement discretion policy for LDTs never applied to tests intended for emergencies, potential emergencies, or material threats declared under Section 564 because false results can have serious implications for disease progression, public health decision-making, and patient care. Instead, after all previous declarations under Section 564, FDA has generally expected LDTs to comply with applicable requirements of the FDCA and FDA regulations. However, FDA has adopted, and may continue to adopt, specific enforcement discretion policies for such tests.
FDA’s draft Enforcement Policy for Certain In Vitro Diagnostic Devices for Immediate Public Health Response in the Absence of a Declaration under Section 564 sets forth FDA’s enforcement policy for “immediate response” tests for use in an emergent situation (i.e., the period of time between detection of the exposure or outbreak and either resolution of the exposure or outbreak or issuance of an applicable Section 564 declaration). Notably, the policy only applies to premarket review requirements and does not extend to tests with home specimen collection or at-home tests.
Under the policy, FDA does not intend to object to the offering of “immediate response” tests when:
- The test is manufactured and offered by laboratory manufacturers meeting certain criteria
- The test has been appropriately validated
- FDA is notified
- Appropriate transparency is provided
- The test is labeled for prescription use only
- There is no applicable Section 564 declaration
If no applicable Section 564 declaration is made within 12 months of the start of an emergent situation, FDA anticipates that the public health rationale for the enforcement policy will no longer apply at that time. FDA would then expect the laboratory manufacturer to cease offering the immediate response test or seek approval/clearance/authorization. FDA does not intend to object to the continued offering of an immediate response test while the premarket submission is prepared, submitted, and under FDA review so long as the laboratory manufacturer submits the submission within 12 months from the date of the first offering of the immediate response test.
FDA’s draft Consideration of Enforcement Policies for Tests During a Section 564 Declared Emergency, when finalized, will describe the factors FDA plans to assess in deciding whether to issue an enforcement policy regarding test manufacturers’ offering of certain devices (i.e., unapproved tests and unapproved uses of approved tests) for the diagnosis of disease or other conditions during a declared emergency. FDA intends to assess, among other things:
- The need for accelerated availability of such tests
- The known or potential risks of such tests
- The availability of appropriate alternative tests that are authorized or approved
- The availability of sufficient mitigations to address risks of false results
What’s Next?
FDA intends to conduct webinars, publish guidance documents, provide templates, and participate in conferences to help laboratories understand and comply with applicable devices. We also think it likely that FDA will increase inspections of laboratories offering IVDs as LDTs where the agency has identified or received concerns regarding their quality or accuracy and will start enforcing the FDCA against laboratories and similar entities perceived to be abusing the agency’s Research Use Only/Investigational Use Only policy or not complying with Investigational Device Exemption (IDE) requirements. As we noted in our Advisory on the October proposed rule, FDA also has identified a number of test types which the agency believes present a higher risk of patient harm when run as LDTs (according to the agency, these include tests such as non-invasive prenatal screening).
Pre-submission meetings, pre-IDE meetings, and other FDA engagement related to data generation, regulatory pathway clarification, and classification will likely increase. Industry will need to closely watch steps FDA takes to lessen the burden of the final rule, such as the noted reclassification process, as well as any potential personnel or structural changes, and future funding requests. As the final rule preamble discussion notes, the alignment of the phaseout policy to coincide with the next round of Medical Device User Fee Amendments reauthorization suggests that the agency understands that it will have to carefully evaluate the burden of this exceptional expansion of FDA authority in terms of protecting the public health while not slowing down the availability of key diagnostic advancements to aid patient care.
We anticipate that laboratories and other affected entities will consider pursuing legal action against the agency, arguing that FDA lacks authority to regulate LDTs and seeking to enjoin the agency from implementing the final rule. The preamble discussion attempts to anticipate and resolve a number of the key challenges raised in comments to the proposed rule, such as important legal questions about FDA authority under the Federal Food, Drug, and Cosmetic Act, interstate commerce concerns, limits on regulation of state-licensed actors, and many other salient issues. FDA clearly is of the view that public health exigencies outweigh the litigation risks, and the final rule phaseout policy and other enforcement discretion positions are sufficient to balance the interests of industry, patients, and the agency.
It remains to be seen what actions Congress may take now that FDA has articulated a final position on this topic. Although Congress has taken an interest in the regulation, and lack thereof, of LDTs, including holding a recent hearing, a legislative solution that would potentially supersede the final rule is uncertain, if not unlikely in the near term. Therefore, given the current state of affairs, it is important for laboratories offering LDTs to begin strategizing on how they will address the final rule and FDA’s phaseout policy.
A version of this article first appeared on Arnold & Porter’s BioSlice Blog.
About The Authors:
 Mahnu Davar co-chairs Arnold & Porter’s life sciences and healthcare regulatory practice. He counsels clients on mission-critical FDA, CLIA, fraud and abuse, and state licensing regulatory matters, including compliance program development and management. He routinely assists multinational companies with sensitive internal investigations, regulatory inspections, product quality and safety issues, data integrity concerns, and government enforcement matters. He also assists medical technology and diagnostics clients with product classification, clearance/approval, and commercial strategy issues.
Mahnu Davar co-chairs Arnold & Porter’s life sciences and healthcare regulatory practice. He counsels clients on mission-critical FDA, CLIA, fraud and abuse, and state licensing regulatory matters, including compliance program development and management. He routinely assists multinational companies with sensitive internal investigations, regulatory inspections, product quality and safety issues, data integrity concerns, and government enforcement matters. He also assists medical technology and diagnostics clients with product classification, clearance/approval, and commercial strategy issues.
 Philip Desjardins is a partner at Arnold & Porter with nearly two decades of experience working with the FDA’s Center for Devices and Radiological Health and serving in leadership roles in Johnson & Johnson’s MedTech businesses. As CDRH associate director for policy, he played a role in formulating the FDA’s current policies on medical devices and drug-device combinations, including legislation, regulations, and enforcement actions. He helped develop and implement CDRH policies in the medtech space on clinical trials and audits. As regulatory counsel, Phil advised FDA leaders on the legal implications of potential compliance actions, pending litigation, enforcing post-market regulatory requirements, and responses to congressional inquiries.
Philip Desjardins is a partner at Arnold & Porter with nearly two decades of experience working with the FDA’s Center for Devices and Radiological Health and serving in leadership roles in Johnson & Johnson’s MedTech businesses. As CDRH associate director for policy, he played a role in formulating the FDA’s current policies on medical devices and drug-device combinations, including legislation, regulations, and enforcement actions. He helped develop and implement CDRH policies in the medtech space on clinical trials and audits. As regulatory counsel, Phil advised FDA leaders on the legal implications of potential compliance actions, pending litigation, enforcing post-market regulatory requirements, and responses to congressional inquiries.
 Abeba Habtemariam is a partner at Arnold & Porter who provides legal, regulatory, and strategic advice to companies that engage in activities regulated by the FDA. She routinely counsels life sciences companies on matters for a range of FDA-regulated products, including drugs, biological products, medical devices, digital health tools, artificial intelligence-enabled software, combination products, and laboratory-developed tests. She has extensive experience advising on medical device clearance and approval strategies, navigating drug regulatory exclusivities, promotional review, and responding to FDA and DOJ enforcement actions.
Abeba Habtemariam is a partner at Arnold & Porter who provides legal, regulatory, and strategic advice to companies that engage in activities regulated by the FDA. She routinely counsels life sciences companies on matters for a range of FDA-regulated products, including drugs, biological products, medical devices, digital health tools, artificial intelligence-enabled software, combination products, and laboratory-developed tests. She has extensive experience advising on medical device clearance and approval strategies, navigating drug regulatory exclusivities, promotional review, and responding to FDA and DOJ enforcement actions.
 Phillip DeFedele is a senior associate at Arnold & Porter. He counsels clients in the life sciences industry on regulatory, transactional, compliance, and enforcement matters. His areas of focus include FDA regulation of drugs, devices, and biologics, fraud and abuse, research and development, advertising and promotion, and compliance programs. Phil assists life sciences companies with regulatory matters throughout the entire product life cycle, including FDA inspections and enforcement and advisory actions as well as matters that are essential for FDA approval, clearance, or licensure.
Phillip DeFedele is a senior associate at Arnold & Porter. He counsels clients in the life sciences industry on regulatory, transactional, compliance, and enforcement matters. His areas of focus include FDA regulation of drugs, devices, and biologics, fraud and abuse, research and development, advertising and promotion, and compliance programs. Phil assists life sciences companies with regulatory matters throughout the entire product life cycle, including FDA inspections and enforcement and advisory actions as well as matters that are essential for FDA approval, clearance, or licensure.
