Why It Is Never Too Early To Consider Tungsten Interactions
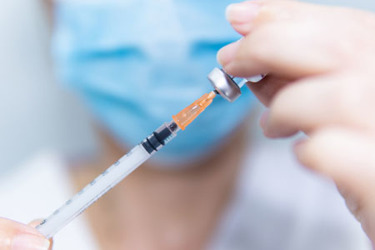
Syringes have a vital role to play in the administration of parenteral therapies and in helping to ensure medication is sterile and free of contaminants. A key challenge to sensitive biotechnological or biologic drugs, especially proteins, is the potential presence of tungsten and tungsten oxides from the syringe forming process. Tungsten oxides comprise a wide range of molecules that can interact differently with biologic drugs, leading to protein unfolding or aggregation, loss of efficacy and unwanted immune reactions in patients, thus jeopardizing product quality and patient safety.
Choosing the wrong container might compromise the stability of the drug product, this may not show up until after Phase 3 stability studies when the final container is used. Evaluating container-drug compatibility earlier in the drug formulation process minimizes patient risk and the risk of making last-minute changes. Formulation or container changes may result in repeat stability testing, which can significantly increase cost and time to market.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Med Device Online? Subscribe today.
