Will Stemless Implants Dominate The Shoulder Arthroplasty Market?
By Yulia Sorokina and Jesse Spicer, iData Research Inc.

Shoulder implant design has been gradually evolving towards shorter implant stems since total shoulder arthroplasty results were first reported over 40 years ago. Reflecting a growing trend of minimally invasive surgeries, stemless arthroplasty is driven by the desire to facilitate smoother revision procedures, and to prevent stem-related complications.
According to a recent market study, the use of stemless implants in shoulder arthroplasty is growing, and it is expected to surpass the use of stemmed implants in Europe by the year 2025. To understand this trend, we can look at multiple factors, including the history, product development, and clinical benefits of stemless implants.
At first glance, the stemless implant is superficially similar to a resurfacing. The main differences are the thicker metaphyseal anchorage, as well as the larger head component that replaces part of the humeral head, rather than just the surface. Therefore, in contrast to resurfacing, stemless arthroplasty allows for glenoid replacement. Stemless shoulder implants do, in fact, have a short stem, although it does not enter the diaphysis of the bone like a stemmed device. Instead, it remains completely in the metaphysis of the humeral neck.
The first stemless arthroplasty product, the T.E.S.S. stemless system, was introduced by Zimmer Biomet in 2004. Since then, various manufacturers have brought their stemless design options into the market. Although stemless arthroplasty is not an entirely new development, with over a decade’s presence in the market, its popularity is much more prominent in Europe than in the United States. In 2015, SIMPLICITI by Wright Medical received approval by the Food and Drug Administration (FDA) and became the first stemless design available in the United States. In Europe, however, the implant’s clinical life began in 2010 in France. Today, there are still only a few options for stemless shoulder implants currently available in the U.S., leaving a large opportunity for manufacturers to take advantage of.
Besides the T.E.S.S. system, Zimmer Biomet’s product portfolio includes two other bone-sparing design options, the Comprehensive Nano, which is the second generation of the T.E.S.S. system, and the Sidus, which entered the European market in 2012. The Comprehensive Nano has a convertible design, which can be utilized in anatomic or reverse arthroplasty. In January 2018, Zimmer Biomet announced that its Sidus Stem-Free Shoulder system had received a FDA clearance.
Another early adaptor of stemless technology was Arthrex, which introduced the Eclipse stemless system in 2005. Following Arthrex, in 2008, Mathys brought its stemless arthroplasty system, Affinis Short, into the European market. Two other companies, FX Solutions and Lima Corporate, launched their stemless products — EASYTECH and Lima Shoulder Modular Replacement (SMR), respectively — in 2015. Addressing the rising stemless implants market trend, these companies have continued investing in the bone-sparing arthroplasty market. Two recent product introductions in Europe include DePuy Synthes’ GLOBAL ICON, as well as Arthrosurface’s OVO Primary Stemless Shoulder system with inlay glenoid. While the product from Arthrosurface was introduced in the U.S. in 2009, the company only announced its plan in 2017 to make the implant commercially available in Europe.
Clinical Studies Prove Case For Stemless Shoulder Implants
The decision to choose stemless options has become easier as more clinical studies prove comparability with their stemmed counterparts. The first stemless shoulder arthroplasty clinical paper was published in 2010 by Huguet et al. The 3-year follow-up study covered 63 patients given the Zimmer Biomet T.E.S.S. implant, and showed an 11.1 percent, 3-year revision rate of the implant. Since then, there have been several short and midterm studies conducted on stemless implants, which showed the comparability of stemless and stemmed arthroplasty outcomes in the up-to-5-year follow ups.
In September 2017, the first long-term study on the effectiveness of stemless implants became available, indicating positive results. The study demonstrated 9-year clinical outcome comparability of stemless shoulder implants to that of third and fourth-generation standard shoulder arthroplasty, as well as significant improvement in patient satisfaction.
While overall clinical evidence supports the use of bone-sparing implants, there also has been research outlining some less favorable outcomes of stemless arthroplasty. For example, a 2016 study, conducted on 241 patients, demonstrated higher incidences of deep-joint infection after stemless arthroplasty, when compared to conventional stemmed arthroplasty.
Advantages And Disadvantages Of Stemless Implants
As a less-invasive shoulder arthroplasty option, stemless implants are associated with a number of advantages over conventional shoulder arthroplasty. Stemless prosthesis allows bone preservation and is associated with less blood loss, as well as with a lower risk of both intra and postoperative fractures. From the procedural point of view, stemless implants normally require shorter surgery times, and their removal is much easier in the case of a follow up revision procedure. Clinical studies show a low prevalence of component loosening in stemless implants. Another advantage of stemless implants is in posttraumatic joint reconstruction cases, where bone deformities make the implantation of stemless implants a more suitable option.
However, stemless implants also are associated with some disadvantages. This type of shoulder arthroplasty design might be unsuitable for patients with different types of metabolic bone disease and/or poor bone structure. Considering that there is currently no objective measure to determine the quality of the bone and its suitability for stemless arthroplasty before the actual procedure, stemless implant arthroplasty is associated with a certain level of risk.
Future Developments And Opportunities
As seen in recent market trends, the multiple advantages of bone-sparing implants outweigh their drawbacks, and stemless shoulder implants are gaining increased acceptance in Europe. With more clinical data accumulated over time and long-term clinical studies outlining positive outcomes, the level of surgeon skepticism towards stemless implants has been declining across Europe. However, acceptance of stemless implants is following different patterns across different European countries.
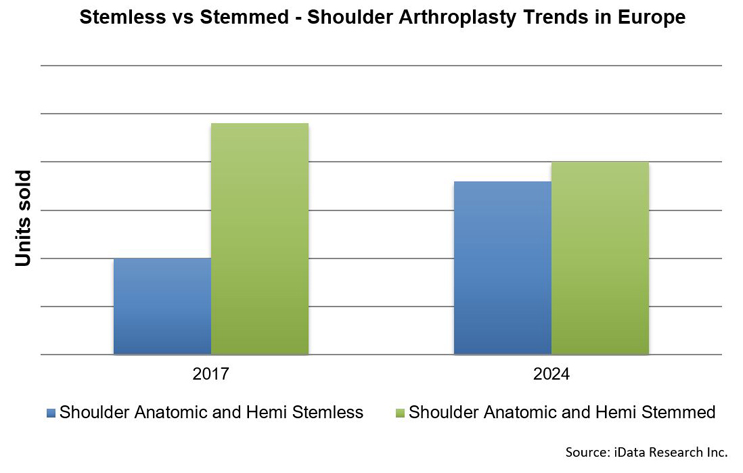
Both total and hemi arthroplasty are seeing a distinctive shift from stemmed to stemless implant designs. The combined number of total stemless implant units and hemi arthroplasty in Europe is anticipated to almost double from 2017 to 2024, and it is expected to surpass stemmed by 2025. Furthermore, over the next decade, manufacturers are expected to bring more stemless implant options to the market for reversed arthroplasty. In 2017, there were very few approved stemless poducts available for reversed shoulder replacements — a great opportunity for manufacturers to capitalize on.
About The Authors
Yulia Sorokina is a Research Analyst at iData Research. She was the lead researcher on the latest research covering the Small Joints Orthopedic Device Market in Europe and has a history of other research projects with iData in Orthopedics.
Jesse Spicer is an Analyst Manager at iData Research. He has been involved in numerous orthopedic market research projects over many years, and now leads a team involved in that market and many others.
About iData Research
iData Research is an international market research and consulting firm focused on providing market intelligence for the medical device, dental and pharmaceutical industries.
References
- European Market Report Suite for Small Bone and Joint Orthopedic Devices – 2018. iData Research Inc.
- European Market Report Suite for Small Bone and Joint Orthopedic Devices – 2015. iData Research Inc.
- Athwal, George S. (2016). Spare the Canal: Stemless Shoulder Arthroplasty Is Finally Here. Commentary on an Article by R. Sean Churchill, MD, et al. “Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System. A Prospective Two-Year Multicenter Study”. The Journal of Bone & Joint Surgery, 98 (3), 28. Doi: 10.2106/JBJS.15.01350
- Huguet D., DeClercq G., Rio B., Teissier J., Zipoli B. (2010). TESS Group Results of a New Stemless Shoulder Prosthesis: Radiologic Proof of Maintained Fixation and Stability after a Minimum of Three Years’ Follow-up. Journal of Shoulder and Elbow Surgery, 19(6):847–852. doi: 10.1016/j.jse.2009.12.009
- R. Sean Churchill, George S. Athwal (2016). Stemless Shoulder Arthroplasty—Current Results and Designs. Current Reviews in Musculoskeletal Medicine, 9(1): 10–16. Doi: 10.1007/s12178-016-9320-4
- Hawi N., Magosch P., Tauber M., Lichtenberg S., Habermeyer P. (2017). Nine-ear Outcome after Anatomic Stemless Shoulder Prosthesis: Clinical and Radiologic Results. Journal of Shoulder and Elbow Surgery, 26(9), 1609-1615. Doi: 10.1016/j.jse.2017.02.017
