World's First Remote Enrollment In Acute Stroke Clinical Trial
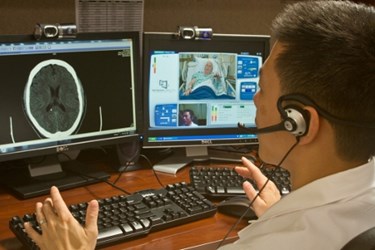
Researchers at the University of Texas Health Science Center (UTHealth) in Houston have successfully completed the first acute stroke clinical trial that enrolled and remotely treated patients via telemedicine. The team believes they have found a feasible solution to overcome clinical trial enrollment issues by using technology to broaden the pool of potential participants.
A study published last year in JAMA Internal Medicine reported that 10 percent of clinical trials are not completed due to a failure to recruit a sufficient number of participants. A major contributing factor to this failure is geographical distance — patients are either unable or unwilling to travel to clinical trial sites.
“Innovative solutions are required to address geographic barriers to access,” said the study authors.
Tzu-Ching Wu, director of the telemedicine program at UTHealth, explained that geographical distance is a particular problem for acute stroke clinical trials because of the time-sensitive nature of the treatments. Patients may be willing, but if the experimental treatment needs to be implemented within hours of the onset of symptoms, travel to another hospital is not always possible.
“One of the main drawbacks of conducting clinical trials for stroke is that we traditionally are limited to patients who arrive at large stroke centers that have the expertise to treat stroke quickly to minimize damage to the brain,” said Wu in a UTHealth press release.
According to Wu, telemedicine is an excellent resource for stroke trials because researchers can enroll from multiple hospitals and “widen the pool” of participants.
In a study published in Annals of Clinical and Translational Neurology, a team of researchers led by Andrew Barreto enrolled a total of 10 patients to test the efficacy of using transcranial Doppler ultrasound in combination with tissue plasminogen activator (tPA). Six of the patients were from different hospitals.
“Eligible patients were successfully identified, consented, randomized, and received therapy/placebo at the spoke hospitals under real-time direction by hub trialists via telemedicine,” reported the authors of the study.
The researchers found that there were no procedural delays or safety events, and the study authors expressed their enthusiasm over the potential of telemedicine in clinical trials and stroke treatment.
“This new use of telemedicine could substantially increase access and opportunities for stroke patients at rural and community hospitals to receive promising new therapies,” they said.
According to the researchers, other studies had used telemedicine to enhance acute stroke clinical trial enrollment, but the UTHealth study was the first to conduct the treatment without first transferring the patient to the main hub.
“Instead of the patients having to be taken to the mother ship, we brought the study to the patient. The implications are enormous,” said Wu in the press release.
Image source: University of Texas Health Science Center at Houston
