3D Printing In Medicine: 4 Questions That Need To Be Answered
By Jim Pomager, Executive Editor
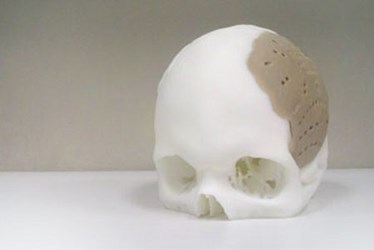
Imagine this scenario.
A patient lies on a surgical bed in a futuristic operating room, having recently been scanned by a cluster of sensors overhead. Across the room, a 3D printer puts the finishing touches on a custom implant that was designed by the patient’s surgeon, who used the scan results to modify a 3D blueprint downloaded from the Internet. When the device is finished printing, the surgeon implants it using surgical instruments 3D printed for that specific procedure. At the conclusion of the surgery, the doctor discards the used instruments in a special bin for materials to be recycled for future 3D printing use.
Sound like science fiction?
“Actually, we’re not that far off,” Michael Drues, president of Vascular Sciences (and Med Device Online guest columnist), informed attendees during an MD&M West presentation titled Future Medical Applications in 3D Printing: Clinical Benefits, Regulatory Issues, and Manufacturing Challenges.
Drues presented several case studies that illustrated 3D printing’s incredible potential in the medical space. One example was from the University of Michigan, where doctors 3D printed a custom, bioresorbable implant for a critically ill infant, based on CT scans of the child’s trachea and bronchus (and after receiving emergency FDA clearance).
Another came from Haiti, where efforts are underway to enable clinics — which often lack the most basic of supplies — to 3D print umbilical cord clamps and other simple medical devices. With 3D printers and an Internet connection, these clinics could conceivably download complex models and produce their own medical supplies, allowing them to circumvent corrupt and inefficient local import systems. “It’s a quintessential case of on-demand, hyperlocal manufacturing,” Drues said. “If we can do this in Haiti, we should be able to do it anywhere.”
An on-demand, 3D printing production model would solve all manner of problems for medical device makers and their customers, according to Drues. Hospitals would be spared from inventory, ordering, storage, and reuse (cleaning and sterilization) headaches. They could simply use a device once and throw it away (or perhaps recycle it). Manufacturers would no longer have to worry about things like packaging, sterility, shelf-life, and stability.
As great as this sounds, Drues cautioned that the medical device industry has some serious work to do before it can realize such utopian visions (and $1-billion market projections). “What we are doing or even talking about now in 3D printing is absolutely boring and represents the lowest of the low-hanging fruit compared to what we could and hopefully will be doing in the future,” he said.
So what’s holding 3D printing back from achieving a more exciting future in medical applications? Drues identified four primary questions the medical device industry must answer before 3D printing can reach its full potential.
1. How do we test 3D printed devices?
3D printed implants and other personalized devices pose a major testing challenge for the industry. When devices are tailor-made for a specific person, as in the University of Michigan example, how do you conduct clinical trials for them? “How do we test such devices, if at all?” Drues asked. “Is the benchmark to have no unforeseen post-op issues after one year and full resorption in three years?” Perhaps personalized medicine — with its one-product-for-one-patient approach — demands more personalized trials of some sort. Or maybe we need to validate the process related to custom 3D printed devices, not the final products.
2. What is the regulatory pathway?
“How do we regulate 3D printing in medicine?” Drues asked the audience. In the University of Michigan example, the custom 3D printed device was granted an emergency use exemption by the FDA, but what happens the next time the researchers want to use a similar custom implant on a different patient? Did the initial device serve as a precedent? Will the researchers be able to seek another emergency use, humanitarian device (HDE), or custom device (CDE) exemption? What if we want to offer custom devices to the masses? Will it need to go through the 510(k) process, or will it be a premarket approval (PMA) situation?
Drues wondered whether some 3D printed devices — those that incorporate a combination of device, drug, and/or biologic — are even true “medical devices” in the regulatory sense of the term. A new approval pathway is probably in order, he reasoned, one that will prompt better collaboration between multiple centers within the FDA (CDRH, CDRH, CBER). The good news is there are signs that the FDA is already reorganizing and rethinking its approach to the complex regulatory issues posed by 3D printing and other emerging technologies.
3. Do we need a new business model?
What happens when these two emerging trends ultimately intersect: 1) the movement toward home-based medical devices and 2) the increasing availability of home-based 3D printers? Drues foresees a “brave new world of medical devices,” where patients download and print their own medical devices at home. Where will today’s device manufacturers fit into that equation? It’s time to start thinking about new business models, Drues said. It may soon be time to start selling designs, not devices.
4. Will biofriendly materials become available?
When asked to identify the biggest challenge facing the technology in the medical device industry, most of the 3D printing vendors whose booths I visited at MD&M West had the same reply: biocompatible plastic materials. While some of the materials commonly used in 3D printing could be described as biocompatible, they are not implantable-grade — they can come into contact with the outside of the body but not the inside. “We may have biocompatible materials now,” Drues said, “but we need biofriendly materials.”
Clearly, there are many questions that still need to be answered before 3D printing can enter the medical mainstream. And in all likelihood, more will arise as the technology continues to advance. Drues brought up regenerative medicine and the 3D printing of human organs, a hot research area right now. If you think creating a test protocol or regulatory path for 3D printed implants is complicated, imagine how difficult it will be for 3D printed organs.
Refusing to end his talk on a pessimistic note, Drues encouraged the device industry to embrace both the risk and the opportunity that 3D printing offers, no matter how daunting the hurdles may seem. “Nothing is impossible,” he said. “The impossible just takes a little longer.”
How are you using 3D printing at your company? Are there other hurdles to adoption that you’ve identified? Share your experiences and opinions in the Comments section below.
Image credit: Oxford Performance Materials (OPM)
