FDA Approves Weight Loss Device That Drain Stomach Contents

By Jof Enriquez,
Follow me on Twitter @jofenriq
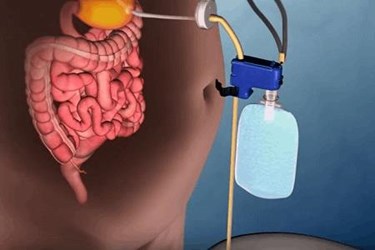
The U.S. Food and Drug Administration (FDA) has approved a unique weight loss device that drains a portion of stomach contents before they are absorbed, helping obese patients to successfully shed and keep off the extra pounds.
Developed by Aspire Bariatrics, the AspireAssist system consists of an endoscopically placed gastric tube connected to a disk-shaped port valve flush against the skin of the abdomen. About 20 to 30 minutes after eating a meal, the patient attaches the device’s external connector and tubing to the port valve, which opens to drain a portion of the stomach contents into a toilet over 5 to 10 minutes. The company claims that the pump can remove about 30 percent of calories consumed. The device is indicated for obese adults aged 22 and older with a BMI of 35 to 55 for whom conservative and non-surgical weight loss therapies have been unsuccessful.
“The AspireAssist approach helps provide effective control of calorie absorption, which is a key principle of weight management therapy,” said William Maisel, M.D., M.P.H., deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health (CDRH), in a press release. “Patients need to be regularly monitored by their health care provider and should follow a lifestyle program to help them develop healthier eating habits and reduce their calorie intake.”
FDA said that during a clinical trial, 111 patients implanted with the AspireAssist device, who also underwent lifestyle therapy such as diet and exercise, lost an average of 12.1 percent of their total body weight after one year, compared to 3.6 percent for 60 control patients who underwent lifestyle changes alone.
The device automatically shuts off after 115 cycles (approximately five to six weeks of therapy), at which time the patient must return to their doctor to get a replacement part for the device. Frequent medical visits are also needed to adjust the tubing placement as the patient loses weight and abdominal girth. FDA states that AspireAssist is not intended to be used in short duration by moderate-weight patients and is contraindicated for those with stomach ulcers, inflammatory bowel disease, pregnancy or lactation, serious pulmonary, or cardiovascular disease. The agency also specifies that the device should not be used by those with eating disorders, such as bulimia.
According to Today, some have labeled AspireAssist as “assisted bulimia” given the device’s mechanism, but a physician who helped test the device brushed off the criticism.
“There is no such thing as medical bulimia or assisted bulimia,” said Dr. Shelby Sullivan of Washington University in St. Louis. “Patients eat less with this therapy than they did before. People think patients can eat whatever they want and then aspirate it and that’s just not true. It has to be liquid enough and the particles have to be small enough to get through the tube.”
AspireAssist is a viable alternative for obese patients who have failed to lose excess weight after taking prescription weight drugs or implementing lifestyle and behavioral interventions, and who are skeptical or do not qualify for bariatric surgery.
“With less than 1% of the 25 million Americans with BMIs over 35 availing themselves of bariatric surgery each year, there is clearly a need for a non-surgical weight loss procedure that is effective, safe, and reversible. AspireAssist therapy satisfies this need and additionally offers a lower cost solution to the healthcare system,” Christopher Thompson, MD, associate professor of medicine at Harvard Medical School and the director of therapeutic endoscopy at Brigham and Women’s Hospital, said in an Aspire Bariatrics press release.
Last year, FDA approved ReShape Medical’s Integrated Dual Balloon System, another minimally invasive anti-obesity device which works by occupying space within the stomach to help patients feel full faster and longer.
