Graphene-Based Wearable Noninvasively Monitors And Treats Diabetes
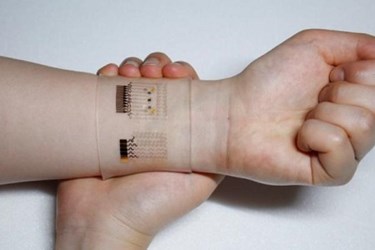
Korean scientists have developed a wearable patch capable of monitoring glucose levels in a patient’s sweat and delivering diabetes medication through an array of microneedles. Because its combination graphene-and-gold sensors can also monitor pH levels and temperature, the device can systematically calibrate itself to provide more accurate readings.
Graphene is the thinnest material that can conduct heat as well as electricity, and it is gaining momentum in the biotech industry, especially among researchers investigating quick-diagnostics and wearables. Certain chemical properties have limited graphene’s biosensing capabilities in the past —especially with glucose — but scientists from the Center for Nanoparticle Research at the Institute for Basic Science (IBS) believe they have overcome these limitations by mixing the material with gold and adding a serpentine layer of gold mesh.
The prototype device fits over the wrist and estimates blood glucose levels by analyzing a sweat sample, which is less invasive than the traditional finger-stick blood sample. The wearable can transmit data wirelessly to the patient’s smart device and provides consistent results after multiple uses.
In a proof-of-concept study published in Nature Nanotechnology, IBS researchers tested the sensing accuracy of the device on two healthy adult men and found a reliable correlation between the device’s readings and those performed with a commercially available glucose monitor.
According to Dae-Hyeong Kim, senior author of the study, the wearable was designed not only to provide monitoring, but to deliver therapeutic treatment as well. If the sensors detect abnormally high levels of glucose, a heat-sensitive array of microneedles releases the drug metformin painlessly into the skin, Kim explained in a press release. Studies demonstrated that the patch was capable of regulating blood glucose levels in diabetic mouse models.
Kim said the entire measurement process took roughly 15 minutes to complete, a process that some experts argue is too long for Type 1 diabetics and impractical for Type 2 diabetics, who don’t test as often as Type 1 patients.
Alongside the IBS study, Nanotechnology Nature published an editorial by Richard Guy, a pharmacologist from the University of Bath. According to Guy, the device proposed by IBS scientists would be unable to administer a therapeutic dose of metformin in humans without being substantially larger than the prototype. Furthermore, the device lacks the ability to test and treat glucose levels that are dangerously low.
Joel Zonszein, director of the Clinical Diabetes Center at Montefiore Medical Center in New York City, told U.S. News & World Report that he was impressed with the concept, calling noninvasive monitoring and treatment “the holy grail of diabetes.” However, he also warned that “there are many barriers to be overcome” before the technology is commercially feasible.
Image credit: IBS
