Latin America Is Becoming The Testing Ground For AI-Enabled Medical Device Clinical Trials
By Julio G. Martinez-Clark, CEO, bioaccess

Artificial-intelligence (AI)-enabled medical devices are proliferating globally, with the U.S. FDA tallying almost 1,000 cleared products as of mid-2024.¹ Latin America (LATAM) is simultaneously modernizing its regulatory frameworks — led by Brazil’s Law 14.874/24 and ANVISA’s (the Brazilian health authority) predetermined change control plan (PCCP)-ready software rules — to become a strategic testing ground for these technologies.² This article explains why LATAM’s demographic diversity, cost profile, and accelerating digital health infrastructure make it an ideal region for AI medical device clinical trials. This article also outlines practical steps for sponsors to navigate country-specific regulations, manage cross-border data, and conduct future-proof studies against adaptive algorithm challenges.
The AI Device Boom
As of late 2024/early 2025, the FDA has authorized over 1,000 AI/ML-enabled medical devices..¹ The agency’s December 2024 guidance, Predetermined Change Control Plans for Medical Devices, permits pre-approved algorithm updates, signaling a harmonized life cycle approach to adaptive software.³ Yet manufacturers still struggle with: (a) securing representative data sets, (b) financing iterative validation, and (c) navigating fragmented global regulations that lag AI’s pace of change.⁴
LATAM’s Strategic Advantages
Genetic & Demographic Diversity: LATAM’s 660 million inhabitants represent one of the world’s most genetically admixed populations, offering a single region where device algorithms can be trained on Native-American, European, African, and Asian ancestries.⁵ Clinical-trial diversity guidelines from IMDRF and FDA directly encourage such recruitment.⁶
Cost & Timeline Benefits: Study start-up costs in Brazil, Mexico, and Colombia average 25%–35 % lower than in the U.S.⁷ Parallel ethics-and-regulator reviews under Brazil’s new law reduce first-in-human start-up to ≈ 60 to 90 days, versus ≥ 180 days under EU MDR.² Faster enrollment (15%–20 % quicker than in North America) shortens overall study timelines.⁸
Digital-Health & Telemedicine Growth: LATAM’s remote patient monitoring market is growing at 13.79% CAGR, with Brazil and Mexico deploying AI-supported telehealth platforms to feed de-identified real-world data into post-market algorithm surveillance.⁹
Country-By-Country Regulatory Snapshot
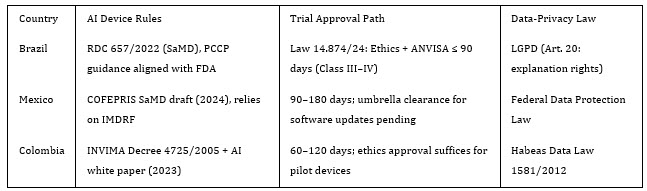
Table 1: Simplified comparison of leading LATAM jurisdictions.
Operational Challenges & Solutions
Cross-Border Data Transfers: High-resolution imaging and continuous physiologic streams quickly exceed national hosting limits. Sponsors should adopt hybrid federated-learning architectures, encrypting at the source and aggregating model weights, rather than raw data to comply with LGPD, GDPR, and HIPAA concurrently.¹⁰
Algorithm Drift & PCCPs: Adaptive AI can drift when exposed to new patient cohorts. Draft PCCPs filed with ANVISA or COFEPRIS (Mexico’s health authority) should include: (1) drift trigger thresholds, (2) locked-model rollback plans, and (3) cloud-based audit logs.³ The International Medical Device Regulators Forum’s (IMDRF) 2025 GMLP principles provide a 10-point checklist (e.g., representative data, continuous performance monitoring) that LATAM regulators are beginning to reference.¹¹
Infrastructure Gaps: Bandwidth constraints in parts of Peru and Bolivia necessitate edge-AI device options with on-device inference and delayed batch uploads.12 Sponsors can de-risk by selecting urban hubs for imaging endpoints and leveraging mobile study coordinators for rural data capture.
A Best Practice Framework
- Country Selection – Map risk class and intended use against each regulator’s AI readiness; prioritize Brazil for early feasibility and Mexico for pivotal enrollment.
- Regulatory Dossier – Submit a unified core file (IMDRF ToC) with country-specific annexes; include algorithm explainability reports and bias-mitigation plans.
- Data Governance Plan – Use federated analytics, PCCP-defined updates, and ISO-27001-certified hosting in-region; obtain explicit consent per Brazil’s LGPD Article 6.
- CRO Partnership – Engage hybrid global-local CRO teams with SaMD experience; ensure bilingual site staff are comfortable with eSource and AI-driven adjudication.
- Post-Market Surveillance – Integrate remote monitoring feeds; push over-the-air model improvements under PCCP without requiring new submissions.
Future Outlook
IMDRF’s 2025 N88 and N81 documents will likely be absorbed into Brazil’s and Colombia’s software rules within two years, harmonizing PCCP content and software-specific risk characterizations.¹¹ Venture capital is already flowing: LATAM AI medical device funding topped $600 million in 2024, up 40% year over year.13 Remote patient monitoring revenue in the region is projected to exceed $1.4 billion by 2029.⁹ Sponsors that move early can secure algorithm-ready data sets and first-mover reimbursement pathways.
Conclusion
Latin America offers a rare trifecta for AI-enabled medical device trials: diverse data, cost efficiency, and increasingly AI-savvy regulators. By adopting federated data strategies, PCCP-aligned protocols, and local partnerships, manufacturers can accelerate validation while future-proofing against global regulatory shifts. LATAM is no longer an emerging option — it's a strategic imperative for AI device developers seeking robust, generalizable evidence at speed and scale.
References
- MedTech Dive. “AI in medtech is booming. Track new devices here.” 2025.
- Government of Brazil. Law 14.874/24. 2024.
- FDA. “Final Guidance on Predetermined Change Control Plans for AI Devices.” Dec 2024.
- JAMA Netw Open. “Generalizability of FDA-Approved AI-Enabled Devices.” 2025.
- Ministry of Health Brazil. “Ethnic and Genetic Diversity Report.” 2024.
- IMDRF. “Good Machine Learning Practice Guiding Principles.” 2025.
- Brazilian Clinical Research Association. “Cost Efficiency in Medical Device Trials.” 2023.
- Clinical Leader. “Latin America’s Landscape for Medtech Clinical Trials.” 2022.
- MarketDataForecast. “LATAM Remote Patient Monitoring Market, 2024-2029.” 2024.
- DLA Piper. “Cross-Border Guide to Clinical Trials & Privacy.” 2024.
- IMDRF/AIML WG/N88 FINAL & SaMD WG/N81 FINAL. 2025.
- IDB. “Digitalization and Telehealth Infrastructure in LATAM.” 2024.
- BCG. “AI and Machine Learning Stake a Claim on Medtech.” 2025.
About The Author:
 Julio G. Martinez-Clark is co-founder and CEO of bioaccess, a market access consultancy that works with medical device companies to help them do early-feasibility clinical trials and commercialize their innovations in Latin America. Julio is also the host of the LATAM Medtech Leaders podcast: A weekly conversation with Medtech leaders who have succeeded in Latin America. He has a bachelor's degree in electronics engineering (BSEE) and a master's degree in business administration (MBA).
Julio G. Martinez-Clark is co-founder and CEO of bioaccess, a market access consultancy that works with medical device companies to help them do early-feasibility clinical trials and commercialize their innovations in Latin America. Julio is also the host of the LATAM Medtech Leaders podcast: A weekly conversation with Medtech leaders who have succeeded in Latin America. He has a bachelor's degree in electronics engineering (BSEE) and a master's degree in business administration (MBA).
