Meeting The Unique Design Requirements For Leadless Pacemaker Electrical Components
Today, millions of people around the world rely on pacemakers to help regulate their heart’s rhythm. A traditional pacemaker usually consists of a pulse generator that is about the size of a tea bag and implanted under the skin near the collarbone, and a wire, or lead, that runs through a blood vessel to the heart. The end of the lead has an electrode on it that touches the heart wall to deliver electrical impulses. However, in the last decade, innovations in pacemaker technology have led to the introduction of a new style of pacemaker, known as the leadless pacemaker, that is about 1/10th the size of a traditional pacemaker, or about the size of a vitamin (Figure 1).
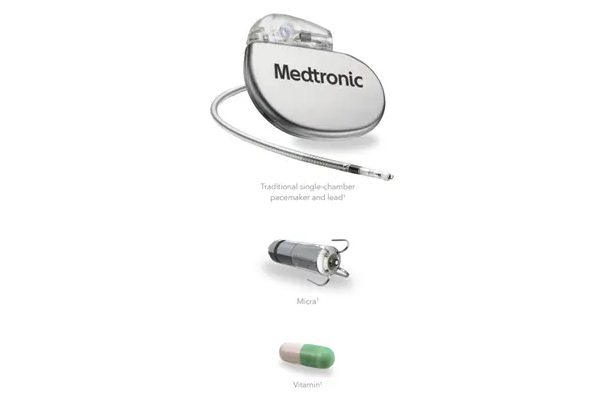
Figure 1. A comparison from Medtronic of the size of a traditional pacemaker with a lead, the Micra leadless pacemaker, and a vitamin. Source.
This tiny leadless pacemaker is a self-contained generator and electrode system, which means it does not require leads and why it is referred to as a leadless pacemaker. This device is designed to be implanted directly in the heart through the femoral vein, which eliminates many of the common complications associated with traditional pacemakers that stem from the leads that permanently run through a patent’s veins into the heart. It is important to note that leadless pacemakers cannot replace a traditional pacemaker as these devices will only work for patients who require single-chamber ventricular pacing such as those who have Bradycardia, which is a slow heart rate that is usually below 60 beats per minute.
Selecting the Right Electrical Components for Miniaturized Medical Devices
Since leadless pacemakers are incredibly small and are implanted directly in a patient’s heart, the electrical components, such as capacitors, used in these devices must be miniaturized while maintaining high reliability. These are not requirements all electrical component manufacturers can meet, but Knowles Precision Devices can.
We have a lot of experience designing components for a variety of medical device manufacturers and understand that manufacturers of medical implantable devices are highly regulated by regulatory bodies such as the International Standards Organization (ISO) and the US Food and Drug Administration (FDA). We also know there are tight controls placed on the design, development, and manufacture of these devices that must be met by the suppliers of the component parts as well. Therefore, we work closely with medical device innovators to create source control drawings (SCD) that govern every aspect of the capacitors we supply. We also have an in-depth understanding of the two main SCD specifications for medical components – MIL-PRF-55681 and MIL-PRF-123.
We also understand that beyond meeting reliability and other regulatory requirements, there many other factors steering capacitor choice, including material, leakage resistance, stability, and price. This is why we supply a wide-variety of high-reliability capacitors that can meet the exact specifications you need for medical implantable devices. Learn more about our offerings by downloading our High-Reliability Products brochure.
