Package Integrity Testing Of Sterile Instruments
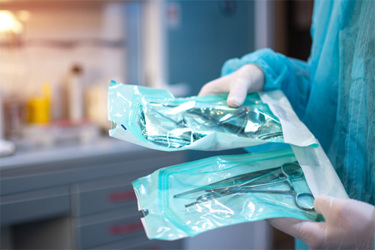
Maintaining the sterility of surgical instruments is critical for patient safety and successful outcomes. Packaging failures, such as occlusions, channel leaks, and weak seals, can compromise sterilization and allow contaminants to enter, posing serious risks in the operating room. To address these challenges, advanced inspection technologies provide quantitative, non-destructive methods for verifying package integrity. Vacuum decay testing measures pressure changes in a vacuum chamber to detect leaks in porous trays and non-porous pouches, while airborne ultrasound uses high-frequency sound waves to identify seal weaknesses in flexible packaging. Both methods offer precise, reliable detection without damaging the contents, ensuring compliance with ISO 11607 and FDA standards.
Explore how these techniques safeguard sterility and streamline quality assurance processes.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Med Device Online? Subscribe today.
