Understanding The Differences Between Pacemakers And ICDs

For more than 3 million people in the United States, pacemakers and implantable cardioverter defibrillators (ICDs) are life-changing technology they rely on. While both devices are implantable medical devices designed to improve the quality of life for people with heart arrhythmia, a condition where the heart beats irregularly, each devices serve a different purpose.
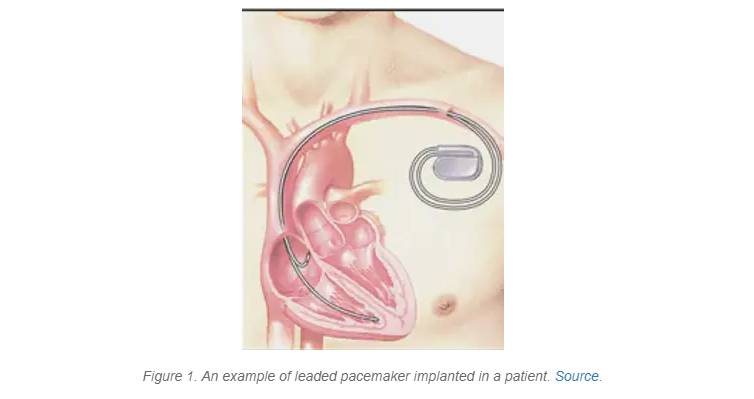
A pacemaker, shown in Figure 1, is an implantable medical device designed to help patients maintain a normal heartbeat and rhythm. The small device is placed under the patient’s skin in their upper chest and consists of a computer that senses when the heart beats at the wrong speed, or out of rhythm. When the pacemaker detects that the heart is out of rhythm, it sends out low-energy electrical pulses to return the heartbeat to a steady rhythm and rate.
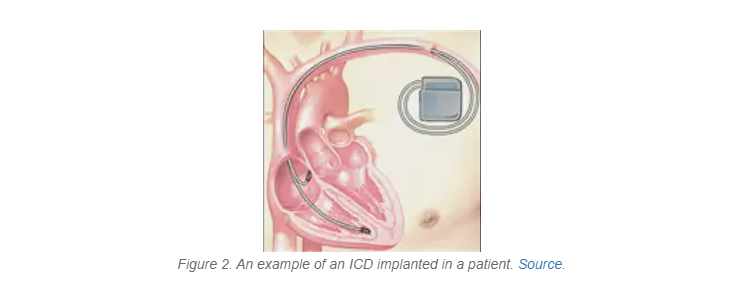
Rather than aiding in maintaining a regular heartbeat, an ICD is designed to prevent or stop a potentially dangerous arrhythmia that could lead to sudden cardiac arrest by using low- or high-energy electric shocks (Figure 2). Like a pacemaker, an ICD is implanted under a patient’s skin and contains a computer that tracks heart rate and rhythm. The key difference between the two devices is that with an ICD, if the patient’s heart beats way too fast or is very out of rhythm, the ICD will send out a shock to get it back into rhythm. Some ICDs can also function like a pacemaker and send out a signal when the heart rate gets too slow as well.
While pacemakers and ICDs serve different functions, there are a lot of similarities between these two implantable medical devices. Since both devices are extremely important to the lives of the patients that require them, it is essential that the small circuit boards inside these devices, which contain very tiny electronics such as capacitors, are built using high-reliability (Hi-Rel) electrical components.
The Challenges of Designing Electrical Components for Life-Sustaining Technology
Since both pacemakers and ICDs are implanted inside the body, these devices must be made as small as possible. Today, medical device designers are focused on innovating device designs to further reduce size and are currently working on the development of new leadless pacemakers that are about 1/10th the size of a traditional pacemaker. Therefore, the electrical components such as capacitors used in these devices must continue to be miniaturized, which can be very challenging.
A great option to help reduce capacitor size is to use multi-layer ceramic capacitors (MLCCs). Since multiple layers can be built in the same capacitor with an MLCC, the result is a single capacitor that provides a capacitance level equivalent to using multiple SLCs connected in parallel. While this multi-layer design is slightly thicker (taller) than an SLC, it decreases the overall footprint needed for a capacitor to achieve the higher capacitance required by pacemakers and ICDs.
In addition to needing increasingly smaller capacitors, the capacitors used in these devices must also be highly reliable. While high reliability sounds like it could be a subjective term, in the medical industry, high reliability has a very specific meaning – the component must be designed to maintain consistent excellence in quality and safety over long periods of time.
This is not an easy task as these components must be put through rigorous testing and extensive screening using established military specifications (MIL-SPECs) to prove reliability. For medical components, MIL-PRF-55681 and MIL-PRF-123 are the most common screening specifications used. At a high level, MIL-PRF-55681 defines a mid-K stable dielectric designated as BX while MIL-PRF-123 covers the general requirements for high reliability, general purpose (BX and BR), and temperature stable (BP and BG) ceramic dielectric fixed capacitors, through-hole, and SMDs. Screening using MIL-PRF-123 provides an increased level of reliability over MIL-PRF-55681 as the screening specifications are more stringent.
Knowles Precision Devices Is Here To Help
At Knowles Precision Devices we are well-versed in guiding device manufacturers through the MLCC selection process and working together to perform the testing and screening required for your device in-house at our facilities in the United States. We have decades of experience manufacturing high-reliability MLCCs suitable for implantable medical devices such as ICDs and Pacemakers. This means you can be confident that our components will produce the level of reliability and safety necessary for being on the circuit boards of your life-critical implantable medical devices.
We also understand that beyond meeting the miniaturization and Hi-Rel requirements for capacitors described in this post, there many other factors steering capacitor choice, including material, leakage resistance, stability, and price. This is why we supply a wide-variety of Hi-Rel capacitors that can meet the exact specifications you need for medical implantable devices. From performing functions such as basic electric charge storage to electromagnetic interference (EMI) and high-frequency noise filtering to absorbing and smoothing signals, we have highly reliable options for your device.
Learn more about our Hi-Rel product offerings by downloading our High-Reliability Products brochure.
