Australian Company BVT Raises $18 Million For Bionic Eye Clinical Trial
By Jof Enriquez,
Follow me on Twitter @jofenriq
Melbourne-based Bionic Vision Technologies (BVT) has raised US $18 million from Hong Kong-based investors China Huarong International Holdings and State Path Capital Limited to proceed with clinical studies testing the company's bionic eye for patients with retinitis pigmentosa, a degenerative condition that is the most common cause of inherited blindness.
BVT's bionic eye is based on intellectual property first developed in 2008 by Bionic Vision Australia (BVA), a consortium made up of University of Melbourne, the University of New South Wales, the Bionics Institute, Centre for Eye Research Australia, Commonwealth Scientific and Industrial Organisation's (CSIRO's) Data 61 research unit, the Royal Victorian Eye & Ear Hospital, Western Sydney University, and the Australian College of Optometry. They now become shareholders alongside the Chinese investors in BVT as it transitions into a commercial business.
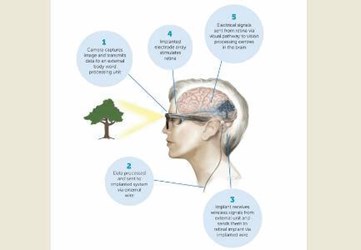
The bionic eye system works by turning images captured by smart glasses into signals transmitted to a retinal implant, whose electrodes stimulate the surviving retinal cells in patients with retinal degeneration. Then, those cells send electrical signals to vision processing centers in the brain to interpret the information.
The Australian Research Council had given a five-year AU$50 million Special Research Initiative grant to develop the bionic eye up to the prototype phase; it was successfully tested in three patients between 2012-2014. BVT plans to use the fresh investment to proceed with next-stage clinical trials in Melbourne. Compared to periodic clinic evaluations in the initial trial, new studies will monitor patients' mobility and independence as they go through everyday activities.
“The funding will propel this Australian technology into clinical trials in coming months as we work towards securing regulatory approval and a commercial launch in key markets where loss of vision is a significant medical burden,” said BVT Executive Chairman Robert Klupacs in a news release. “There is currently no treatment for conditions such as retinitis pigmentosa and our new investors recognise BVT has developed a world-leading solution with potential to make a significant impact patient’s sight and lifestyle.”
Other companies racing against BVT in commercializing a bionic eye include California-based Second Sight Medical, who is developing the Orion I Visual Cortical Prosthesis that is set for human trials this year, and French technology company Pixium Vision, which is trying to commercialize a light-powered eye implant. Google’s parent company Alphabet also has a patent application for an intraocular device powered by an external antenna.
BVT claims it has the edge over competitors due to a superior surgical approach using a natural anatomical pocket called the suprachoroidal space, the stability of its device, and a unique vision-processing software that improves the patient’s experience. The company's bionic eye was a finalist in the 2013 Eureka Awards and the 2013 Melbourne Awards, according to Business Insider.
