NORDSON MEDICAL: ABOUT US
Your Single-Source Partner
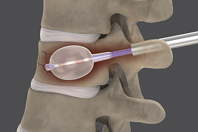
Nordson MEDICAL (Nasdaq: NDSN) is a global expert in the design, development, and manufacturing of complex medical devices and component technologies. As a single-source partner, we enable our customers to save costs, speed time to market, and simplify supply chain management.
Our expertise includes fluid management components, polymer solution-cast cannulae, heat shrink tubing, medical balloons, medical tubing, biomaterial delivery devices, nitinol components, and inflation devices. We also provide surgical technologies such as myocardial protection systems, aortic punches, vascular loops, safety valves, ophthalmology catheters, and infusion therapy products.
We work with companies at any point in the product lifecycle, from concept to launch and beyond. With our flexible business model, we can provide a solution that meets the scope and scale of any project to bring innovative ideas to life.
Click here to shop online: https://www.nordsonmedical.com/Shop/
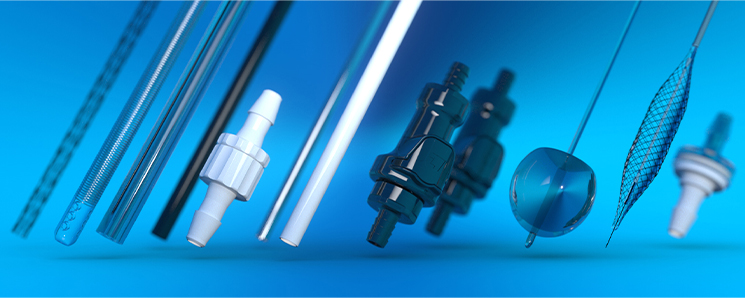
Components & Technologies
- Cannulae - Polymer Solution Casting
- Inflation Devices
- Pressure-Relief Valves
- Inflation Products
- MPS® Myocardial Protection Systems
- Aortic Punches
- Vascular Loops
- Safety Valves
- LacriCATH® Balloon Catheters
- Infusion Therapy Products
- Medical Balloons
- Heat Shrink Tubing
- Polyimide Tubing
- PTFE Tubing
- Extruded Tubing
- Reinforced Tubing
- Luer Fittings
- Tube Fittings
- Quick Connect Couplings
- BioProcessing Fittings
- Blood Pressure Fittings
- Threaded Fittings
- Stopcocks
- Check Valves
- Needlefree Swabable Valves
- Tubing
- Biomaterial Delivery Devices
- Bone Graft Delivery Devices
- Nitinol Components
Unmatched Service
We enable our customers’ success through superior products and services by industry-leading innovation, quality, reliability, and responsiveness. Nordson MEDICAL can provide the detailed support you need from idea to market. Along with our technical team, we also offer online prototyping tools and project accelerators to help you design and develop to get your innovation to market faster.
Markets We Serve
- BioProcessing
- Cardiovascular
- Diagnostics
- Drug Delivery
- Electrophysiology
- Gastrointestinal
- Intravenous
- Inflation & Volumetric Containers
- Infusion Therapy
- Neurovascular
- Non-Medical
- Analytical Instrumentation
- Food and Beverage
- Inkjet
- Ophthalmology
- Patient Care
- Blood Pressure
- Dialysis
- Neuraxial
- Peripheral Vascular
- Robotic Surgery
- Surgical
- Gastointestinal
- Interventional
- Neurovascular
- Ophthalmic
- Orthopedic
- Urology
- Structural Heart
- Hemodialysis
Quality Assurance
We hold ourselves to the highest standards of quality. With over 50 years of medical device experience, we have developed a thorough understanding of the complex regulatory environment. Every product is manufactured to your specifications, and in full compliance with FDA guidelines, at one of our ISO-certified facilities. We host over 100 audits per year from customers and regulatory bodies.
Certifications & Registrations
- ISO 13485 and 14971 risk management certified
- FDA registered and compliant with the Quality System Regulation
- Class I, II, and III medical devices, including Premarket Approval (PMA) products
- Class 7 and Class 8 cleanroom-controlled environments
- US FDA 21 CFR Part 820 compliance
- Canadian Medical Device Conformity Assessment System compliance
- Medical Device Single Audit Program (MDSAP)
CONTACT INFORMATION
Nordson MEDICAL
29 Northwestern Drive
Salem, NH 03079
UNITED STATES
FEATURED ARTICLES
 PolyPeel™ Peelable Polyester Heat Shrink Tubing
PolyPeel™ Peelable Polyester Heat Shrink Tubing
PET heat shrink is a strong barrier for medical devices, but difficult to remove. Peelable polyester heat shrink tubing is designed to overcome PET’s shortcomings.
-
Peelable polyester heat shrink tubing offers a PFAS-free, easy-to-remove alternative for catheter manufacturing, ensuring precision, safety, and efficiency with ultra-thin walls, high dielectric strength, and versatile applications.
-
Electrophysiology and pulsed field ablation device development demands precision, speed, and reliability. Multi-component suppliers streamline manufacturing, reduce risk, and accelerate innovation, ensuring faster market entry and consistent quality.
-
A virtual start-up partnered with Nordson MEDICAL for complete design, development, and manufacturing of a catheter-based device for TAVR patients, leading to CE mark and acquisition.
-
A medical device company worked to redesign a critical component and consolidate extrusion and secondary operations. The project was successfully completed in six months.
-
Nordson MEDICAL helped a startup develop a balloon catheter for atrial fibrillation, leading to its acquisition by a major medical technology company. They offer a range of components and tools, and specialize in medical device design and manufacturing.
-
A multinational medical device company worked to redesign an introducer sheath for a neurovascular microcatheter. After successful prototypes, the design was completed in 8 weeks.
-
A multinational medical device company successfully worked to redesign a component used in cardiovascular surgery.
-
Discover products for accurate and precise delivery of biomaterials including tissue sealants, hemostats, adhesion barriers, and other autologous or synthetic biomaterials required in surgical applications.
-
Explore the benefits of using balloon catheters in medical procedures and their positive impact on patient's lives and healthcare outcomes.
-
This white paper aims to explore the significance and challenges of the standardization of plastic luer fittings and the importance in ensuring the quality and safety of medical devices.
-
The use of single-use plastic luer fittings in medical settings, medical devices, and patient care is an example where the sustainability versus safety debate arises. This white paper aims to explore this debate and offer insights into the challenges and opportunities that lie ahead.
-
This paper aims to demonstrate the cost effectiveness of plastic luer fittings and how understanding the factors that contribute to their cost can help users select a cost-effective option that meets their performance requirements.
-
With its shape memory properties, nitinol wire is a highly suitable material for a range of medical applications. Given its exceptional mechanical and thermal properties, the demand for nitinol products is on the rise. This paper delves into the process of forming and manipulating nitinol wire to create customized components.
-
Discover how breakthroughs in multilayer tubing extrusion technology enable next-generation catheter-based medical devices.
-
Most medical tubing specifications comprise a drawing of a tube with the material, dimensions, and tolerances. However, parameters associated with the production of the tubing are also key.
-
This application note provides medical device engineers ideas for applications that are ideally suited for ultra-thin-walled PET heat shrink tubing. Applications explored in this paper include variable-stiffness catheters, protective covering/coating and bundling, reinforcement, tube making and printing, and electrical insulation.
-
Polyimide is a versatile polymer with a wide range of desirable properties that can be used to great effect in many different microdiameter tubing applications. Thanks to the novel film-cast tubing process, medical device engineers can take full advantage of this stable, durable polymer in ways that might not be obvious at first glance.
-
This case study explores three medical device design challenges and how Nordson MEDICAL devised optimal solutions with the innovative use of the film-cast process with polyimide and other polymers.
-
This article describes just a few of the many innovative applications for balloon catheters today. Other applications include the use of implantable balloons for weight loss, heat transfer balloons for induced hypothermia treatment, and even balloons filled with bone cement to stabilize broken bones.